Exam 2: Atomic Structure and Radioisotopes
Exam 1: Matter, Energy, and Measurement91 Questions
Exam 2: Atomic Structure and Radioisotopes107 Questions
Exam 3: Ionic and Covalent Compounds95 Questions
Exam 4: Molecular Geometry, Polarity, and Intermolecular86 Questions
Exam 5: Chemical Quantities and Introduction to Reactions88 Questions
Exam 6: Chemical Reactions: Energy, Rates, and Equilibrium87 Questions
Exam 7: Changes of State and Gas Laws99 Questions
Exam 8: Mixtures, Solution Concentrations, and Diffusion97 Questions
Exam 9: Acids and Bases, Ph, and Buffers83 Questions
Exam 10: Introduction to Organic Chemistry:111 Questions
Exam 11: Alcohols, Phenols, Thiols, Ethers, and Amines69 Questions
Exam 12: The Carbonyl Containing Functional Groups70 Questions
Exam 13: The Common Organic Reactions in Biochemistry82 Questions
Exam 14: Carbohydrates: Structure and Function97 Questions
Exam 15: Lipids: Structure and Function106 Questions
Exam 16: Proteins: Structure and Function134 Questions
Exam 17: Nucleotides and Nucleic Acids109 Questions
Exam 18: Energy and Metabolism115 Questions
Select questions type
Effective dose measurements take into account the ______ of a type of radiation.
(Multiple Choice)
4.9/5  (37)
(37)
According to the periodic table, which element is found in period 2, group 5A?
(Multiple Choice)
4.7/5  (27)
(27)
In which of the following reactions is the missing particle an alpha particle?
(Multiple Choice)
4.9/5  (41)
(41)
According to the graph, what is the half-life in years of 226Ra? 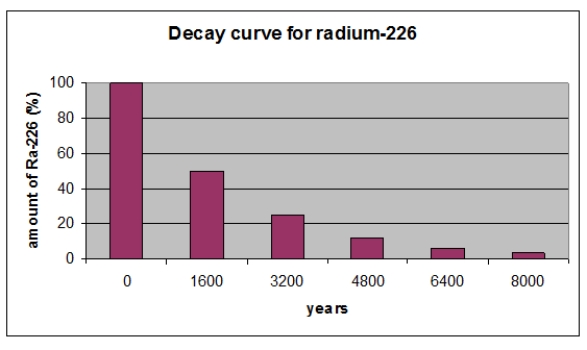
(Multiple Choice)
4.9/5  (29)
(29)
According to the graph, radioactive decay is a(n)_________ in radioactivity as a function of time. 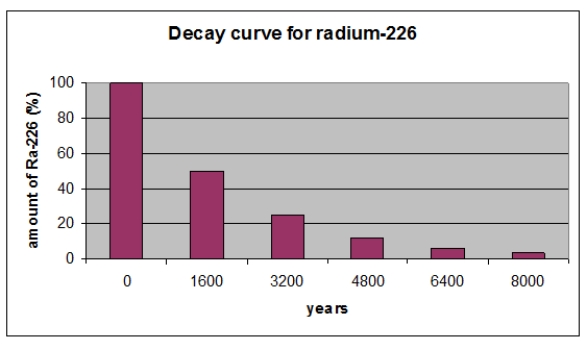
(Multiple Choice)
4.8/5  (44)
(44)
According to the periodic table, what types of elements are in group 1A?
(Multiple Choice)
4.8/5  (34)
(34)
Which statement BEST interprets the statement below? The LD50 for radiation is an acute dose of 3-4 Sv.
(Multiple Choice)
4.9/5  (37)
(37)
_____ is an important component of the immune system as well as required by many enzymes.
(Multiple Choice)
4.7/5  (34)
(34)
According to the periodic table, how many valence electrons do the elements in the third row have?
(Multiple Choice)
4.9/5  (47)
(47)
According to the periodic table, what types of elements are in group 7A?
(Multiple Choice)
4.9/5  (36)
(36)
According to the periodic table, how many valence electrons do the elements in group 7A have?
(Multiple Choice)
4.8/5  (38)
(38)
Which of the following describes a benefit of using Sieverts instead of grays to measure the quantity of radiation that a patient has received?
(Multiple Choice)
4.7/5  (37)
(37)
Showing 21 - 40 of 107
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)