Exam 2: Atomic Structure and Radioisotopes
Exam 1: Matter, Energy, and Measurement91 Questions
Exam 2: Atomic Structure and Radioisotopes107 Questions
Exam 3: Ionic and Covalent Compounds95 Questions
Exam 4: Molecular Geometry, Polarity, and Intermolecular86 Questions
Exam 5: Chemical Quantities and Introduction to Reactions88 Questions
Exam 6: Chemical Reactions: Energy, Rates, and Equilibrium87 Questions
Exam 7: Changes of State and Gas Laws99 Questions
Exam 8: Mixtures, Solution Concentrations, and Diffusion97 Questions
Exam 9: Acids and Bases, Ph, and Buffers83 Questions
Exam 10: Introduction to Organic Chemistry:111 Questions
Exam 11: Alcohols, Phenols, Thiols, Ethers, and Amines69 Questions
Exam 12: The Carbonyl Containing Functional Groups70 Questions
Exam 13: The Common Organic Reactions in Biochemistry82 Questions
Exam 14: Carbohydrates: Structure and Function97 Questions
Exam 15: Lipids: Structure and Function106 Questions
Exam 16: Proteins: Structure and Function134 Questions
Exam 17: Nucleotides and Nucleic Acids109 Questions
Exam 18: Energy and Metabolism115 Questions
Select questions type
How does computed tomography (CT)differ from standard x-ray imaging?
(Multiple Choice)
4.9/5  (30)
(30)
What sort of information do the units used in this table take into account? 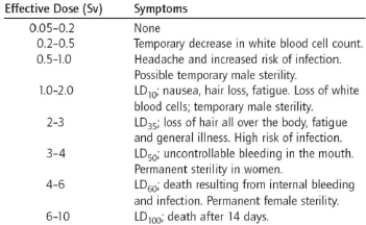
(Multiple Choice)
4.9/5  (30)
(30)
Radioisotopes used in medicine typically have short half-lives.Which of the following statements BEST describes the reason for this?
(Multiple Choice)
4.7/5  (30)
(30)
_____ are the subatomic particles that have the smallest mass.
(Multiple Choice)
4.8/5  (37)
(37)
_____ make up the majority of compounds found in living organisms.
(Multiple Choice)
4.8/5  (41)
(41)
What is the relationship between the energy and the wavelength of light?
(Multiple Choice)
4.9/5  (32)
(32)
What type of radiation is emitted when U-235 undergoes radioactive decay? 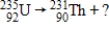
(Multiple Choice)
4.8/5  (27)
(27)
What sort of protection should be used when working with gamma emitters?
(Multiple Choice)
4.7/5  (33)
(33)
_____ is an important component of hemoglobin.Without this protein, tissues become starved of oxygen, and fatigue and shortness of breath results.
(Multiple Choice)
4.8/5  (30)
(30)
The process in which a nucleus spontaneously breaks down by emitting radiation is known as
(Multiple Choice)
4.8/5  (35)
(35)
The time that it takes a macroscopic sample of a radioisotope to decay to one-half its original activity is known as the
(Multiple Choice)
4.9/5  (37)
(37)
Which element is depicted in this drawing of a neutral atom? 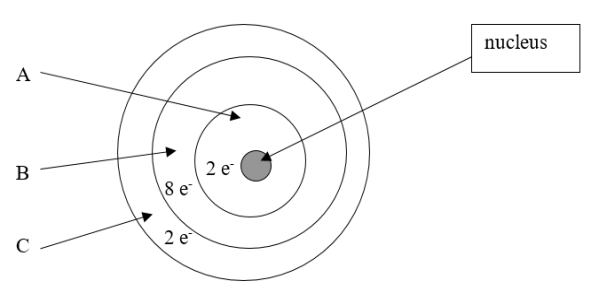
(Multiple Choice)
4.8/5  (33)
(33)
According to the periodic table, how many energy levels do the elements in the third row have?
(Multiple Choice)
4.9/5  (34)
(34)
What is the mass number of an atom of oxygen with seven neutrons?
(Multiple Choice)
4.9/5  (39)
(39)
According to the periodic table, what types of elements are in group 2A?
(Multiple Choice)
4.8/5  (29)
(29)
According to the periodic table, the atomic number of potassium (K)is ______.
(Multiple Choice)
4.7/5  (28)
(28)
Which of the following radioisotope would be LEAST likely to be used in a medical application?
(Multiple Choice)
4.7/5  (30)
(30)
Showing 41 - 60 of 107
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)