Exam 18: A Macroscopic Description of Matter
Exam 1: Concepts of Motion52 Questions
Exam 2: Kinematics in One Dimension59 Questions
Exam 3: Vectors and Coordinate Systems33 Questions
Exam 4: Kinematics in Two Dimensions50 Questions
Exam 5: Force and Motion30 Questions
Exam 6: Dynamics I: Motion Along a Line46 Questions
Exam 7: Newtons Third Law43 Questions
Exam 8: Dynamics Ii: Motion in a Plane20 Questions
Exam 9: Work and Kinetic Energy66 Questions
Exam 10: Interactions and Potential Energy55 Questions
Exam 11: Impulse and Momentum43 Questions
Exam 12: Rotation of a Rigid Body116 Questions
Exam 13: Newtons Theory of Gravity50 Questions
Exam 14: Fluids and Elasticity72 Questions
Exam 15: Oscillations49 Questions
Exam 16: Traveling Waves51 Questions
Exam 17: Superposition51 Questions
Exam 18: A Macroscopic Description of Matter46 Questions
Exam 19: Work, Heat, and the First Law of Thermodynamics96 Questions
Exam 20: The Micromacro Connection41 Questions
Exam 21: Heat Engines and Refrigerators44 Questions
Exam 22: Electric Charges and Forces26 Questions
Exam 23: The Electric Field32 Questions
Exam 24: Gausss Law41 Questions
Exam 25: The Electric Potential40 Questions
Exam 26: Potential and Field57 Questions
Exam 27: Current and Resistance32 Questions
Exam 28: Fundamentals of Circuits68 Questions
Exam 29: The Magnetic Field83 Questions
Exam 30: Electromagnetic Induction66 Questions
Exam 31: Electromagnetic Fields and Waves52 Questions
Exam 32: Ac Circuits44 Questions
Exam 33: Wave Optics51 Questions
Exam 34: Ray Optics60 Questions
Exam 35: Optical Instruments52 Questions
Exam 36: Relativity49 Questions
Exam 37: The Foundations of Modern Physics7 Questions
Exam 38: Quantization45 Questions
Exam 39: Wave Functions and Uncertainty18 Questions
Exam 40: One-Dimensional Quantum Mechanics32 Questions
Exam 41: Atomic Physics41 Questions
Exam 42: Nuclear Physics65 Questions
Select questions type
A weather balloon contains 12.0 m3 of hydrogen gas when the balloon is released from a location at which the temperature is 22.0°C and the pressure is 101 kPa. The balloon rises to a location where the temperature is -30.0°C and the pressure is 20.0 kPa. If the balloon is free to expand so that the pressure of the gas inside is equal to the ambient pressure, what is the new volume of the balloon? Assume that in both cases the hydrogen gas is in thermal equilibrium with the outside air.
(Multiple Choice)
4.8/5  (33)
(33)
The coefficient of volume expansion of olive oil is 0.68 × 10-3 K-1. A 1.00-L glass beaker is filled to the brim with olive oil at room temperature. The beaker is placed on a range and the temperature of the oil and beaker increases by 25°C. As a result, 0.0167 L of olive oil spill over the top of the beaker. What is the coefficient of linear expansion of the glass?
(Multiple Choice)
4.8/5  (39)
(39)
Sometimes an experiment requires a certain pure gas to be used at reduced pressure. One way to achieve this is to purchase a sealed glass container filled with the gas, and to introduce the gas into a vacuum by attaching the glass container to the vacuum chamber and breaking the tip of the glass container using a metallic bean and a magnet. If the volume of the glass container is 1.0 L and it is at a pressure of 1.0 × 105 Pa and if the vacuum chamber has a volume of 2.0 L, what will the pressure be after the gas, which is to be assumed to be an ideal gas, is released into the vacuum chamber and the temperature has returned to its original value? (Note that the glass container remains part of the system.)
(Multiple Choice)
4.9/5  (41)
(41)
A cold trap is set up to cause molecules to linger near the suction in a vacuum system. If the cold trap has an effective volume of 0.200 L and is maintained at 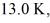 how many molecules are in it at 10.0 Pa of pressure? (Avogadro's number is 6.022 × 1023 molecules/mol, and the universal gas constant is 8.314 J/mol ∙ K. Assume the behavior of an ideal gas.)
how many molecules are in it at 10.0 Pa of pressure? (Avogadro's number is 6.022 × 1023 molecules/mol, and the universal gas constant is 8.314 J/mol ∙ K. Assume the behavior of an ideal gas.)
(Multiple Choice)
4.8/5  (31)
(31)
A rod has a length 2.00000 m at 10.0°C. The length of the rod increases to 2.00060 m when the temperature increases to 30.0°C. What is the coefficient of linear expansion of the material from which the rod is made?
(Multiple Choice)
4.9/5  (44)
(44)
The interior of a refrigerator has a volume of 0.600 m3. The temperature inside the refrigerator in 282 K, and the pressure is 101 kPa. If the molecular weight of air is 29 g/mol, what is the mass of air inside the refrigerator? The ideal gas constant is R = 8.314 J/mol ∙ K = 0.0821 L ∙ atm/mol ∙ K.
(Multiple Choice)
4.7/5  (36)
(36)
Showing 41 - 46 of 46
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)