Exam 18: A Macroscopic Description of Matter
Exam 1: Concepts of Motion52 Questions
Exam 2: Kinematics in One Dimension59 Questions
Exam 3: Vectors and Coordinate Systems33 Questions
Exam 4: Kinematics in Two Dimensions50 Questions
Exam 5: Force and Motion30 Questions
Exam 6: Dynamics I: Motion Along a Line46 Questions
Exam 7: Newtons Third Law43 Questions
Exam 8: Dynamics Ii: Motion in a Plane20 Questions
Exam 9: Work and Kinetic Energy66 Questions
Exam 10: Interactions and Potential Energy55 Questions
Exam 11: Impulse and Momentum43 Questions
Exam 12: Rotation of a Rigid Body116 Questions
Exam 13: Newtons Theory of Gravity50 Questions
Exam 14: Fluids and Elasticity72 Questions
Exam 15: Oscillations49 Questions
Exam 16: Traveling Waves51 Questions
Exam 17: Superposition51 Questions
Exam 18: A Macroscopic Description of Matter46 Questions
Exam 19: Work, Heat, and the First Law of Thermodynamics96 Questions
Exam 20: The Micromacro Connection41 Questions
Exam 21: Heat Engines and Refrigerators44 Questions
Exam 22: Electric Charges and Forces26 Questions
Exam 23: The Electric Field32 Questions
Exam 24: Gausss Law41 Questions
Exam 25: The Electric Potential40 Questions
Exam 26: Potential and Field57 Questions
Exam 27: Current and Resistance32 Questions
Exam 28: Fundamentals of Circuits68 Questions
Exam 29: The Magnetic Field83 Questions
Exam 30: Electromagnetic Induction66 Questions
Exam 31: Electromagnetic Fields and Waves52 Questions
Exam 32: Ac Circuits44 Questions
Exam 33: Wave Optics51 Questions
Exam 34: Ray Optics60 Questions
Exam 35: Optical Instruments52 Questions
Exam 36: Relativity49 Questions
Exam 37: The Foundations of Modern Physics7 Questions
Exam 38: Quantization45 Questions
Exam 39: Wave Functions and Uncertainty18 Questions
Exam 40: One-Dimensional Quantum Mechanics32 Questions
Exam 41: Atomic Physics41 Questions
Exam 42: Nuclear Physics65 Questions
Select questions type
1.000 L of water at 20.00°C will occupy what volume if it is heated to 80.00°C? Water has a volume expansion coefficient of 210 × 10-6/°C. (Express your answer to 4 significant figures.)
(Multiple Choice)
4.8/5  (33)
(33)
A sealed container holds 0.020 moles of nitrogen (N2) gas, at a pressure of 1.5 atmospheres and a temperature of 290 K. The atomic mass of nitrogen is 14.0 g/mol. The Boltzmann constant is 1.38 × 10-23 J/K and the ideal gas constant is R = 8.314 J/mol · K = 0.0821 L · atm/mol · K. The mass density of the gas is closest to
(Multiple Choice)
4.8/5  (41)
(41)
A brass rod is 40.1 cm long and an aluminum rod is 79.3 cm long when both rods are at an initial temperature of 0°C. The rods are placed in line with a gap of 0.60 cm between them, as shown in the figure. The distance between the far ends of the rods is maintained at 120.0 cm throughout. The temperature of both rods is raised until the two rods are barely in contact. The coefficients of linear expansion of brass and aluminum are 2.0 × 10-5 K-1 and 2.4 × 10-5 K-1, respectively. The temperature at which contact of the rods barely occurs is closest to 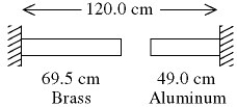
(Multiple Choice)
4.9/5  (30)
(30)
The exterior of a supersonic airplane is made of aluminum, which has a coefficient of linear expansion of 24 × 10-6 K-1. At 15°C, the plane measures 62.1 m in length. When the plane is in flight, friction with the air increases the temperature of the exterior skin to 200°C. What is the change in the length of the outer skin of the plane?
(Multiple Choice)
4.8/5  (34)
(34)
How many moles of water (H2O) molecules are in a 4.00 m3 container at a pressure 8.00 × 105 N/m2 and temperature 600°C? The ideal gas constant is R = 8.314 J/mol ∙ K
= 0)0821 L ∙ atm/mol ∙ K.
(Multiple Choice)
4.8/5  (34)
(34)
A vertical tube that is closed at the upper end and open at the lower end contains an air pocket. The open end of the tube is under the water of a lake, as shown in the figure. When the lower end of the tube is just under the surface of the lake, where the temperature is 37°C and the pressure is 1.0 × 105 Pa, the air pocket occupies a volume of 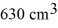 . Suppose now that the lower end of the tube is at a depth of 86 m in the lake, where the temperature is 7.0°C. What is the volume of the air pocket under these conditions? The density of the water in the lake is 1000 kg/m3.
. Suppose now that the lower end of the tube is at a depth of 86 m in the lake, where the temperature is 7.0°C. What is the volume of the air pocket under these conditions? The density of the water in the lake is 1000 kg/m3. 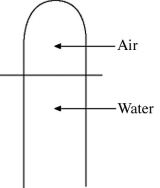
(Essay)
4.7/5  (37)
(37)
An ideal gas is at a pressure 1.00 × 105 N/m2 and occupies a volume 2.00 m3. If the gas is compressed to a volume 1.00 m3 while the temperature remains constant, what will be the new pressure in the gas?
(Multiple Choice)
5.0/5  (32)
(32)
2.0 L of an ideal nitrogen gas (N2) are at 0.00°C and 1.0 atm. The ideal gas constant is
R = 8.314 J/mol ∙ K = 0.0821 L ∙ atm/mol ∙ K, Avogadro's number is 6.022 × 1023 molecules/mol, and the ATOMIC mass of nitrogen is 14 g/mol.
(a) Determine the number of moles of N2.
(b) How many molecules of N2 are present?
(c) What is the mass of this gas?
(Essay)
4.9/5  (38)
(38)
A sealed 26-  tank is filled with 2000 moles of oxygen gas (O2) at an initial temperature of 270 K. The gas is heated to a final temperature of 460 K. The ATOMIC mass of oxygen is 16.0 g/mol, and the ideal gas constant is R = 8.314 J/mol · K = 0.0821 L · atm/mol · K. The final pressure of the gas is closest to
tank is filled with 2000 moles of oxygen gas (O2) at an initial temperature of 270 K. The gas is heated to a final temperature of 460 K. The ATOMIC mass of oxygen is 16.0 g/mol, and the ideal gas constant is R = 8.314 J/mol · K = 0.0821 L · atm/mol · K. The final pressure of the gas is closest to
(Multiple Choice)
4.9/5  (41)
(41)
The figure shows a pV diagram for 0.0066 mol of gas that undergoes the process 1 → 2 → 3. What is the pressure p2. The ideal gas constant is R = 8.314 J/mol ∙ K = 0.0821 L ∙ atm/mol ∙ K. 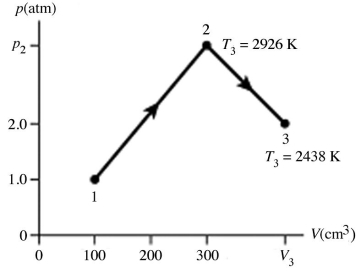
(Multiple Choice)
4.9/5  (38)
(38)
Two identical concrete slabs lie flat and in contact with each other as shown in the figure. If the temperature increases by 40°C, the lower edges opposite the contact edges remained fixed in position, and the lower edges of the contact side remain in contact, at what angle will the slabs be tilted? The coefficient of thermal expansion of the concrete is 10 × 10-6/K. 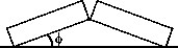
(Multiple Choice)
4.8/5  (50)
(50)
What is the mass density of argon gas at pressure 1.00 × 105 N/m2 and at temperature 300 K? The mean atomic mass of argon is 39.948 g/mol and the ideal gas constant is R = 8.314 J/mol ∙ K = 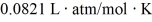 .
.
(Multiple Choice)
4.9/5  (34)
(34)
A sealed 89- 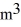 tank is filled with 6000 moles of oxygen gas (O2) at an initial temperature of 270 K. The gas is heated to a final temperature of 350 K. The ATOMIC mass of oxygen is 16.0 g/mol, and the ideal gas constant is R = 8.314 J/mol · K = 0.0821 L · atm/mol · K. The initial pressure of the gas is closest to
tank is filled with 6000 moles of oxygen gas (O2) at an initial temperature of 270 K. The gas is heated to a final temperature of 350 K. The ATOMIC mass of oxygen is 16.0 g/mol, and the ideal gas constant is R = 8.314 J/mol · K = 0.0821 L · atm/mol · K. The initial pressure of the gas is closest to
(Multiple Choice)
4.9/5  (37)
(37)
The figure shows a pV diagram for 8.3 g of nitrogen gas (N2) in a sealed container. The temperature T1 of the gas in state 1 is 79°C. What are (a) the pressure p1 of the gas in state 1 and (b) the temperature T2 of the gas in state 2? The ideal gas constant is R = 8.314 J/mol ∙ K = 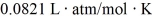 , and the ATOMIC weight of nitrogen is 14 g/mol.
, and the ATOMIC weight of nitrogen is 14 g/mol. 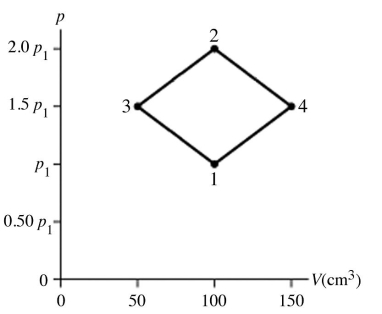
(Multiple Choice)
4.8/5  (31)
(31)
Suppose that a steel bridge, 1000 m long, was built without any expansion joints and that only one end of the bridge was held fixed. What would the difference in the length of the bridge be between winter and summer, taking a typical winter temperature as 0.00°C, and a typical summer temperature as 40°C? The coefficient of thermal expansion of steel is 10.5 × 10-6 K-1.
(Multiple Choice)
4.7/5  (37)
(37)
The figure (not to scale) shows a pV diagram for 1.8 g of helium gas (He) that undergoes the process 1 → 2 → 3. Find the value of V3. The ideal gas constant is R = 8.314 J/mol ∙ K = 0.0821 L ∙ atm/mol ∙ K, and the atomic weight of helium is 4.0 g/mol. 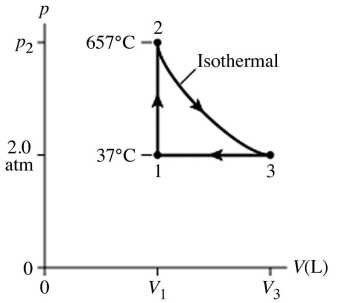
(Multiple Choice)
4.8/5  (34)
(34)
(a) Internal human body temperature is often stated to be normal at 98.6°F. What is this temperature on the Celsius and Kelvin scales?
(b) Gallium boils at 2205°C. What is the corresponding temperature in the Fahrenheit and Kelvin scales?
(c) The boiling point of liquid nitrogen is 77.0 K. What is the corresponding temperature in the Fahrenheit and Celsius scales?
(Essay)
4.8/5  (36)
(36)
A certain metal has a coefficient of linear expansion of 2.00 × 10-5 K-1. It has been kept in a laboratory oven at 325°C for a long time. It is now removed from the oven and placed in a freezer at -145°C. After it has reached freezer temperature, the percent change in its density during this process is closest to
(Multiple Choice)
4.7/5  (36)
(36)
Showing 21 - 40 of 46
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)