Exam 6: Solutions and Colloids
Exam 1: Matter, Energy, and Measurement143 Questions
Exam 2: Atoms134 Questions
Exam 3: Chemical Bonds142 Questions
Exam 4: Chemical Reactions138 Questions
Exam 5: Gases, Liquids, and Solids104 Questions
Exam 6: Solutions and Colloids157 Questions
Exam 7: Reaction Rates and Chemical Equilibrium104 Questions
Exam 8: Acids and Bases198 Questions
Exam 9: Nuclear Chemistry152 Questions
Exam 10: Organic Chemistry71 Questions
Exam 11: Alkanes142 Questions
Exam 12: Alkenes, Alkynes, and Aromatic Compounds184 Questions
Exam 13: Alcohols, Ethers, and Thiols118 Questions
Exam 14: Chirality: the Handedness of Molecules92 Questions
Exam 15: Amines89 Questions
Exam 16: Aldehydes and Ketones102 Questions
Exam 17: Carboxylic Acids115 Questions
Exam 18: Carboxylic Anhydrides, Esters, and Amides117 Questions
Exam 19: Carbohydrates103 Questions
Exam 20: Lipids132 Questions
Exam 21: Proteins128 Questions
Exam 22: Enzymes62 Questions
Exam 23: Chemical Communications: Neurotransmitters and Hormones89 Questions
Exam 24: Nucleotides, Nucleic Acids, and Heredity121 Questions
Exam 25: Gene Expression and Protein Synthesis129 Questions
Exam 26: Bioenergetics: How the Body Converts Food to Energy133 Questions
Exam 27: Specific Catabolic Pathways: Carbohydrate, Lipid, and Protein Metabolism104 Questions
Exam 28: Biosynthetic Pathways67 Questions
Exam 29: Nutrition73 Questions
Exam 30: Immunochemistry132 Questions
Exam 31: Body Fluids72 Questions
Select questions type
Which of the following terms can be used to quantitatively describe the concentration of a solution?
(Multiple Choice)
4.8/5  (47)
(47)
A 750-mL bottle of vodka is labeled as 60 proof. What is its alcohol content?
(Multiple Choice)
4.9/5  (44)
(44)
Which of the following aqueous solutions of Na2SO4 is hypotonic with a 0.15 M NaCl solution?
(Multiple Choice)
4.9/5  (32)
(32)
Consider the following image of a separatory funnel. Which of the following correctly describes the contents of the funnel? 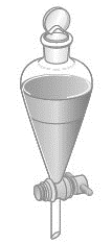
(Multiple Choice)
4.8/5  (39)
(39)
Which ions among Cl-, Na+, NH4+, and NO3- will move toward an anode?
(Multiple Choice)
4.8/5  (37)
(37)
A solution is prepared by dissolving 53.5 grams of ammonium chloride, NH4Cl, in 4000.0 grams of water. What is the boiling point of the solution? (The boiling point elevation constant for water is 0.512°C/mole solute in 1000 g of water.)
(Multiple Choice)
4.9/5  (38)
(38)
Which of the following occurs when red blood cells are placed in an isotonic solution?
(Multiple Choice)
4.8/5  (35)
(35)
Which of the following solutions of glucose, C6H12O6, is isotonic with a 0.15 M NaCl solution?
(Multiple Choice)
4.9/5  (44)
(44)
Which of the following aqueous solutions of Na2SO4 is isotonic with a 0.15 M NaCl solution?
(Multiple Choice)
4.8/5  (39)
(39)
The concentration of lead in a water sample is found to be 3 ppb. To which of the following is this equivalent?
(Multiple Choice)
4.7/5  (38)
(38)
Which term is used to refer to the liquid component of a solid-in-liquid solution?
(Multiple Choice)
4.9/5  (39)
(39)
A chemist has a sample that is known to be a solution. Which of the following processes can be used to separate the components of the solution?
(Multiple Choice)
4.9/5  (37)
(37)
Which of the following is associated with the desalinization of water?
(Multiple Choice)
4.9/5  (40)
(40)
What is the mass of glycine in a 400-g sample of a solution that is 2.5%(w/w)?
(Multiple Choice)
4.9/5  (25)
(25)
Which of the following concentration units will not change if a solution is heated?
(Multiple Choice)
4.9/5  (40)
(40)
A solution is prepared by dissolving 215 grams of methanol, CH3OH, in 1000.0 grams of water. What is the boiling point of the solution? (The boiling point elevation constant for water is 0.512°C/mole solute in 1000 g of water.)
(Multiple Choice)
4.8/5  (42)
(42)
If we wish to prepare 250.0 mL of 0.200 M BaCl2 solution, how much of solid BaCl2 is needed?
(Multiple Choice)
4.9/5  (35)
(35)
Showing 101 - 120 of 157
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)