Exam 14: Chirality: the Handedness of Molecules
Exam 1: Matter, Energy, and Measurement143 Questions
Exam 2: Atoms134 Questions
Exam 3: Chemical Bonds142 Questions
Exam 4: Chemical Reactions138 Questions
Exam 5: Gases, Liquids, and Solids104 Questions
Exam 6: Solutions and Colloids157 Questions
Exam 7: Reaction Rates and Chemical Equilibrium104 Questions
Exam 8: Acids and Bases198 Questions
Exam 9: Nuclear Chemistry152 Questions
Exam 10: Organic Chemistry71 Questions
Exam 11: Alkanes142 Questions
Exam 12: Alkenes, Alkynes, and Aromatic Compounds184 Questions
Exam 13: Alcohols, Ethers, and Thiols118 Questions
Exam 14: Chirality: the Handedness of Molecules92 Questions
Exam 15: Amines89 Questions
Exam 16: Aldehydes and Ketones102 Questions
Exam 17: Carboxylic Acids115 Questions
Exam 18: Carboxylic Anhydrides, Esters, and Amides117 Questions
Exam 19: Carbohydrates103 Questions
Exam 20: Lipids132 Questions
Exam 21: Proteins128 Questions
Exam 22: Enzymes62 Questions
Exam 23: Chemical Communications: Neurotransmitters and Hormones89 Questions
Exam 24: Nucleotides, Nucleic Acids, and Heredity121 Questions
Exam 25: Gene Expression and Protein Synthesis129 Questions
Exam 26: Bioenergetics: How the Body Converts Food to Energy133 Questions
Exam 27: Specific Catabolic Pathways: Carbohydrate, Lipid, and Protein Metabolism104 Questions
Exam 28: Biosynthetic Pathways67 Questions
Exam 29: Nutrition73 Questions
Exam 30: Immunochemistry132 Questions
Exam 31: Body Fluids72 Questions
Select questions type
Identify a class of isomers that has the same connectivity of atoms without any stereocenters.
(Multiple Choice)
4.8/5  (37)
(37)
Lactic acid, shown below, is produced by muscle exercise and can also be found in sour milk. Which of the following statements is true? 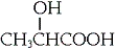
(Multiple Choice)
4.9/5  (40)
(40)
How are the following two carbohydrates related to each other? 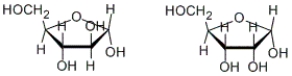
(Multiple Choice)
4.9/5  (40)
(40)
If the specific rotation of the (R)-enantiomer of a compound is −4.8°, what is the specific rotation for the (S)-enantiomer of the compound?
(Multiple Choice)
4.8/5  (35)
(35)
Which of the following represents the enantiomer of the following compound? 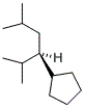
(Multiple Choice)
4.8/5  (33)
(33)
Given the three molecules 1-methylcyclohexanol, trans-2-methylcyclohexanol, and trans-4-methylcyclohexanol, which of the following statements is true?
(Multiple Choice)
4.8/5  (32)
(32)
Consider the following two ball-and-stick models. Elements other than hydrogen and carbon are labeled. 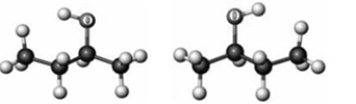 If a solution contains 0.0500 mol of each substance, which of the following describes the solution?
If a solution contains 0.0500 mol of each substance, which of the following describes the solution?
(Multiple Choice)
4.9/5  (40)
(40)
Identify a class of isomers that has different connectivity of atoms.
(Multiple Choice)
4.9/5  (37)
(37)
In order to distinguish the R and S forms of a chiral molecule with a single stereocenter, an enzyme must have binding sites for how many of the groups on the stereocenter of the molecule?
(Multiple Choice)
4.9/5  (36)
(36)
Which of the given structures represents the enantiomer of the following compound? 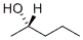
(Multiple Choice)
4.8/5  (34)
(34)
Based on the R,S system, which statement is true of the active enantiomer of ibuprofen?
(Multiple Choice)
4.8/5  (40)
(40)
The specific rotation of a compound is defined as the observed rotation of the compound when its concentration is 1 g/mL in a sample tube that is 10 cm long. If a certain compound is observed to have a specific rotation of +8.0°, what rotation will be observed if the sample concentration is 0.25 g/mL and the sample tube is 20 cm long?
(Multiple Choice)
4.9/5  (35)
(35)
How many stereocenters are present in 2,4-dichloro-3-hexanol?
(Multiple Choice)
5.0/5  (40)
(40)
What are the R/S configurations at the stereocenters of the following compounds? 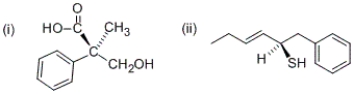
(Multiple Choice)
4.7/5  (44)
(44)
Which of the following three isomeric alcohols is chiral: 2,2-dimethyl-1-butanol, 2-methyl-2-pentanol, and 2-methyl-3-pentanol?
(Multiple Choice)
4.9/5  (33)
(33)
Which of the following is the correct order of priorities in the R,S system?
(Multiple Choice)
4.8/5  (41)
(41)
Showing 21 - 40 of 92
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)