Exam 7: Acids, Bases, and Equilibrium
Exam 1: Science and Measurements81 Questions
Exam 2: Atoms and Elements79 Questions
Exam 3: Compounds82 Questions
Exam 4: An Introduction to Organic Compounds75 Questions
Exam 5: Reactions90 Questions
Exam 6: Gases, Solutions, Colloids, and Suspensions104 Questions
Exam 7: Acids, Bases, and Equilibrium91 Questions
Exam 8: Organic Reactions Hydrocarbons, Carboxlic Acids, Amines, and Related Compounds77 Questions
Exam 9: Organic Reactions 2-Alcohols, Ethers, Aldehydes, and Ketones85 Questions
Exam 10: Carbohydrates84 Questions
Exam 11: Lipids and Membranes90 Questions
Exam 12: Peptides, Proteins, and Enzymes86 Questions
Exam 13: Nucleic Acids99 Questions
Exam 14: Metabolism84 Questions
Select questions type
If a wine has fermented beyond the step that produces alcohol, it becomes acidic, making the wine taste ___.
(Short Answer)
4.7/5  (35)
(35)
In your own words, describe how the HPO42-/H2PO4- buffer system stabilizes the pH inside a cell.

(Short Answer)
4.9/5  (32)
(32)
Which of the following is not a common characteristic of a base?
(Multiple Choice)
4.8/5  (26)
(26)
____ is a condition in which the pH of blood is below the normal range.
(Short Answer)
4.8/5  (42)
(42)
Which of the following is the conjugate acid of the bicarbonate ion, HCO3-?
(Multiple Choice)
4.9/5  (35)
(35)
Whenever an equilibrium constant, Keq, has a value greater than 1, which of the following statements is true at equilibrium?
(Multiple Choice)
4.9/5  (47)
(47)
In the following equation, identify the acid, base, conjugate acid, and conjugate base.

(Short Answer)
5.0/5  (35)
(35)
Calculate the pH of a solution containing 0.15 moles KOH dissolved in enough water to produce 2 liters of solution.
(Multiple Choice)
4.9/5  (33)
(33)
A is red, B is colorless, and C is yellow. Initially the system is in equilibrium and has an orange color.
 What color will the system become if B is added?
What color will the system become if B is added?
(Short Answer)
4.8/5  (36)
(36)
For a buffer to continue to work effectively, the pH of a buffer has to be close to the pKa of the conjugate acid.
(True/False)
4.8/5  (40)
(40)
Which of the following is not a common characteristic of an acid?
(Multiple Choice)
4.7/5  (32)
(32)
Increasing the concentration of a ___ at equilibrium will drive the system toward the reactants.
(Short Answer)
4.9/5  (33)
(33)
Which of the following is the conjugate base of the bicarbonate ion, HCO3-?
(Multiple Choice)
4.7/5  (35)
(35)
15.00 mL of 0.100 M NaOH is required to completely neutralize 25.00 mL of an HCl solution. What is the concentration of the HCl solution?
(Multiple Choice)
4.7/5  (38)
(38)
Calculate the pH of solution produced by dissolving 0.001 moles of HNO3 in a liter of water. Assume complete dissociation.
(Multiple Choice)
4.9/5  (33)
(33)
The equation:
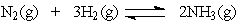 has the following equilibrium constant expression:
has the following equilibrium constant expression:
(Multiple Choice)
4.7/5  (36)
(36)
Showing 61 - 80 of 91
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)