Exam 7: Acids, Bases, and Equilibrium
Exam 1: Science and Measurements81 Questions
Exam 2: Atoms and Elements79 Questions
Exam 3: Compounds82 Questions
Exam 4: An Introduction to Organic Compounds75 Questions
Exam 5: Reactions90 Questions
Exam 6: Gases, Solutions, Colloids, and Suspensions104 Questions
Exam 7: Acids, Bases, and Equilibrium91 Questions
Exam 8: Organic Reactions Hydrocarbons, Carboxlic Acids, Amines, and Related Compounds77 Questions
Exam 9: Organic Reactions 2-Alcohols, Ethers, Aldehydes, and Ketones85 Questions
Exam 10: Carbohydrates84 Questions
Exam 11: Lipids and Membranes90 Questions
Exam 12: Peptides, Proteins, and Enzymes86 Questions
Exam 13: Nucleic Acids99 Questions
Exam 14: Metabolism84 Questions
Select questions type
Calculate the [OH-] in an aqueous solution when the [H3O+] is 1.2 x 10-2M.
(Multiple Choice)
4.7/5  (32)
(32)
The ethylammonium ion, CH3CH2NH3+ has a pKa of 10.81. It reacts with water to form ethylamine, CH3CH2NH2 and H3O+ as shown below.
 Which of the following statements is true at pH 7?
Which of the following statements is true at pH 7?
(Multiple Choice)
4.8/5  (46)
(46)
Hydrogen sulfide ion, HS-, can react differently depending on the acidity of the solution in which it is present.
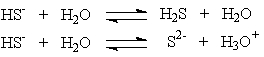 These two solutions show that the
These two solutions show that the
(Multiple Choice)
4.7/5  (31)
(31)
When we talk about pH we are referring to the concentration of hydronium ions present. If the concentration of hydronium in a solution is 1.0 x 10-8 M, what is the hydroxide ion concentration?
(Multiple Choice)
4.7/5  (25)
(25)
In the reaction below:
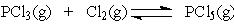 Increasing the concentration of Cl2 will ___ at equilibrium.
Increasing the concentration of Cl2 will ___ at equilibrium.
(Multiple Choice)
4.8/5  (32)
(32)
A base is a compound that produces an ion with the formula ___ in aqueous solution and an acid is a compound that produces the ___ ion in aqueous solution, which is also written as the hydronium ion (___).
(Short Answer)
4.9/5  (32)
(32)
The conjugate acid of the hydrogen sulfate ion (HSO4-) is ___.
(Short Answer)
4.8/5  (27)
(27)
Which of the following are the strong acids? List all.
HF, HNO3, HCN, CH3COOH, H2SO4
(Short Answer)
4.8/5  (35)
(35)
A solution in which the concentration of H+ is greater than the concentration of OH- will
(Multiple Choice)
4.9/5  (43)
(43)
The equation:
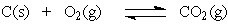 has the following equilibrium constant expression.
has the following equilibrium constant expression.
(Multiple Choice)
4.9/5  (41)
(41)
Calculate the pH of a 0.01 M NaOH solution. Assume complete dissociation.
(Short Answer)
4.8/5  (36)
(36)
The Ka for the reaction of acetic acid and water shown below is 1.8 x 10-5.
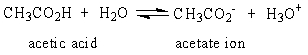 Which of the following statements is true at pH 7?
Which of the following statements is true at pH 7?
(Multiple Choice)
4.9/5  (37)
(37)
One serious effect of cholera is to destroy the hydrogen carbonate ion in the buffer system in the blood. Would the body then suffer from acidosis or alkalosis? Explain.
(Short Answer)
4.8/5  (36)
(36)
What is the pH of a solution in which [H3O]+ is 2.2 x 10-12 M?
(Multiple Choice)
4.8/5  (26)
(26)
What is the molar concentration of hydronium ions in a sample of a soft drink that has a pH of 4?
(Multiple Choice)
4.9/5  (26)
(26)
A is red, B is colorless, and C is yellow. Initially the system is in equilibrium and has an orange color.
 What color will the system become if concentration of A is increased?
What color will the system become if concentration of A is increased?
(Short Answer)
4.7/5  (39)
(39)
Showing 41 - 60 of 91
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)