Exam 7: Acids, Bases, and Equilibrium
Exam 1: Science and Measurements81 Questions
Exam 2: Atoms and Elements79 Questions
Exam 3: Compounds82 Questions
Exam 4: An Introduction to Organic Compounds75 Questions
Exam 5: Reactions90 Questions
Exam 6: Gases, Solutions, Colloids, and Suspensions104 Questions
Exam 7: Acids, Bases, and Equilibrium91 Questions
Exam 8: Organic Reactions Hydrocarbons, Carboxlic Acids, Amines, and Related Compounds77 Questions
Exam 9: Organic Reactions 2-Alcohols, Ethers, Aldehydes, and Ketones85 Questions
Exam 10: Carbohydrates84 Questions
Exam 11: Lipids and Membranes90 Questions
Exam 12: Peptides, Proteins, and Enzymes86 Questions
Exam 13: Nucleic Acids99 Questions
Exam 14: Metabolism84 Questions
Select questions type
A solution of stomach acid contains 1.21 g of HCl in 480.0 mL of solution. Calculate the pH of the solution. Assume complete dissociation.
(Short Answer)
4.8/5  (28)
(28)
A is red, B is colorless, and C is yellow. Initially the system is in equilibrium and has an orange color.
 What color will the system become if it is heated?
What color will the system become if it is heated?
(Short Answer)
4.8/5  (38)
(38)
A sample of orange juice has a pH of 3.44. Its [H3O+] is ___.
(Multiple Choice)
4.8/5  (34)
(34)
The conjugate base of the hydrogen sulfate ion (HSO4-) is ___.
(Short Answer)
4.9/5  (37)
(37)
What is the [H3O+] of an antacid tablet solution with a pH of 8.32?
(Multiple Choice)
4.8/5  (33)
(33)
Which of the following is the conjugate base of the acid, carbonic acid? 
(Multiple Choice)
4.8/5  (37)
(37)
Acids and bases can react with and damage many compounds that are vital to living organisms.
(True/False)
4.7/5  (40)
(40)
When stress is applied to a system at equilibrium such that the equilibrium is disturbed, the reaction proceeds in the direction that counteracts the disturbance. This explanation best describes ___.
(Multiple Choice)
4.8/5  (31)
(31)
A solution of stomach acid contains 1.21 g of HCl in 480.0 mL of solution. Calculate the molarity of the solution. Assume complete dissociation.
(Short Answer)
4.7/5  (34)
(34)
The pH of blood is maintained at 7.35-7.45 by the following buffer system:
 The H2CO3 is produced by the reaction of CO2 with water according to the equation:
The H2CO3 is produced by the reaction of CO2 with water according to the equation:
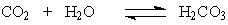 When one exercises, there is increased cellular output of CO2. What effect will this have on the pH of the blood if the excess CO2 is not eliminated?
When one exercises, there is increased cellular output of CO2. What effect will this have on the pH of the blood if the excess CO2 is not eliminated?
(Multiple Choice)
4.8/5  (34)
(34)
What is the concentration of [H3O+] in an aqueous solution when the [OH-] is 5.2 x 10-9 M?
(Multiple Choice)
4.8/5  (32)
(32)
Write the equation illustrating how H2CO3 reacts as an acid with H2O.
(Essay)
4.9/5  (33)
(33)
The carbonate/hydrogen carbonate buffer is responsible for maintaining the pH of human blood in a narrow range between 7.35-7.45. The following equation shows the equilibrium that exists between carbonic acid (H2CO3) and its conjugate base, hydrogen carbonate (HCO3-).
 In you own words, explain what happens when a solution containing hydroxide ions is added to the system and how the buffer prevents the pH from changing.
In you own words, explain what happens when a solution containing hydroxide ions is added to the system and how the buffer prevents the pH from changing.
(Short Answer)
4.9/5  (34)
(34)
A sample of blood has a pH of 7.37, which means that the sample of blood is slightly ___.
(Short Answer)
5.0/5  (45)
(45)
Showing 21 - 40 of 91
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)