Exam 5: Reactions
Exam 1: Science and Measurements81 Questions
Exam 2: Atoms and Elements79 Questions
Exam 3: Compounds82 Questions
Exam 4: An Introduction to Organic Compounds75 Questions
Exam 5: Reactions90 Questions
Exam 6: Gases, Solutions, Colloids, and Suspensions104 Questions
Exam 7: Acids, Bases, and Equilibrium91 Questions
Exam 8: Organic Reactions Hydrocarbons, Carboxlic Acids, Amines, and Related Compounds77 Questions
Exam 9: Organic Reactions 2-Alcohols, Ethers, Aldehydes, and Ketones85 Questions
Exam 10: Carbohydrates84 Questions
Exam 11: Lipids and Membranes90 Questions
Exam 12: Peptides, Proteins, and Enzymes86 Questions
Exam 13: Nucleic Acids99 Questions
Exam 14: Metabolism84 Questions
Select questions type
The coefficients used to balance chemical equations can be interpreted in terms of atoms, moles, or grams.
(True/False)
4.8/5  (26)
(26)
Write and balance the equation for the complete combustion of cyclohexane (C6H12)
(Essay)
4.7/5  (28)
(28)
When properly balanced with whole number coefficients, what is the coefficient on N2 ? 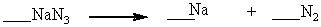
(Multiple Choice)
4.7/5  (43)
(43)
Which set of coefficients properly balance the equation below, going from reactants to products? 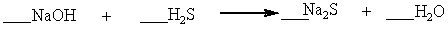
(Multiple Choice)
4.9/5  (40)
(40)
When G for a reaction is +286 kcal, the reaction is spontaneous.
(True/False)
4.8/5  (40)
(40)
Baking soda (NaHCO3) can be used as an antacid. It reacts with and neutralizes stomach acid (HCl). The equation for this reaction is as follows:
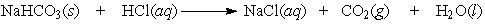 If 10.0 grams of NaHCO3 is added to 10.0 g of HCl, which is the limiting reactant?
If 10.0 grams of NaHCO3 is added to 10.0 g of HCl, which is the limiting reactant?
(Short Answer)
4.8/5  (32)
(32)
Classify the reaction below as involving synthesis, decomposition, single replacement, or double replacement. 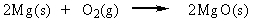
(Multiple Choice)
4.8/5  (30)
(30)
Tums tablets are composed of the substance calcium carbonate. Two tablets have a combined mass of 5.0 grams. Calcium carbonate reacts with and neutralizes excess stomach acid (hydrochloric acid). The products of this reaction are: water, calcium chloride, and carbon dioxide. Calculate the moles of carbon dioxide that will be produced by the reaction of hydrochloric acid with 5.0 grams of calcium carbonate.
(Short Answer)
4.8/5  (27)
(27)
How many moles of aluminum carbonate are required to neutralize 2.68 moles of HCl?

(Short Answer)
4.7/5  (40)
(40)
Ethyne, C2H2, used in welding react with oxygen as shown below.
 Which of the following is about this reaction?
Which of the following is about this reaction?
(Multiple Choice)
4.7/5  (26)
(26)
Lithium is a metal that reacts vigorously with oxygen. Which set of coefficents balance the equation below? 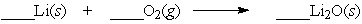
(Multiple Choice)
4.8/5  (32)
(32)
Aluminum will react slowly with the oxygen in the air. When the equation below is balanced, with whole number coefficients, what is the coefficient on aluminum oxide? 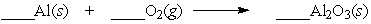
(Multiple Choice)
4.7/5  (35)
(35)
In the reaction energy diagram below, the activation energy is represented by: 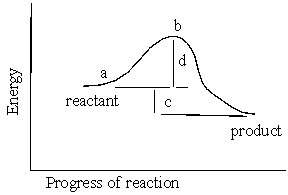
(Multiple Choice)
4.8/5  (36)
(36)
Balance the equation below and determine the number of moles of oxygen required to completely burn 5.00 moles of propane. 
(Multiple Choice)
4.8/5  (38)
(38)
___ lower the ___ of a reaction and therefore increase the reaction rate.
(Multiple Choice)
4.9/5  (35)
(35)
Showing 21 - 40 of 90
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)