Exam 5: Reactions
Exam 1: Science and Measurements81 Questions
Exam 2: Atoms and Elements79 Questions
Exam 3: Compounds82 Questions
Exam 4: An Introduction to Organic Compounds75 Questions
Exam 5: Reactions90 Questions
Exam 6: Gases, Solutions, Colloids, and Suspensions104 Questions
Exam 7: Acids, Bases, and Equilibrium91 Questions
Exam 8: Organic Reactions Hydrocarbons, Carboxlic Acids, Amines, and Related Compounds77 Questions
Exam 9: Organic Reactions 2-Alcohols, Ethers, Aldehydes, and Ketones85 Questions
Exam 10: Carbohydrates84 Questions
Exam 11: Lipids and Membranes90 Questions
Exam 12: Peptides, Proteins, and Enzymes86 Questions
Exam 13: Nucleic Acids99 Questions
Exam 14: Metabolism84 Questions
Select questions type
The catalyst utilized by biological systems for hydrogenation reactions is ___.
(Multiple Choice)
4.9/5  (37)
(37)
How many grams of oxygen (O2) are needed to completely react with 24.0 g of propane (C3H8) according to the equation below? 
(Multiple Choice)
4.9/5  (28)
(28)
Balance the equation and list the coefficients in the balanced equation below, going from reactants to products. 
(Multiple Choice)
4.7/5  (32)
(32)
In the following reaction, 10.0 moles of potassium would require how many moles of bromine? 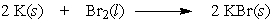
(Multiple Choice)
4.9/5  (24)
(24)
The measured amount of product obtained in any reaction is known as the ___.
(Short Answer)
4.9/5  (31)
(31)
Which set of coefficients properly balance the equation below, going from reactants to products? 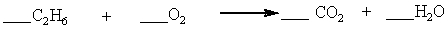
(Multiple Choice)
4.8/5  (42)
(42)
Write and balance the equation for the complete combustion of decane (C10H22)
(Essay)
4.9/5  (38)
(38)
For the following organic reactions, identify the reaction as an oxidation or reduction:
a)
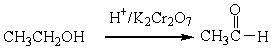 b)
b)
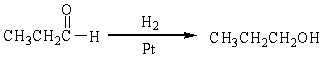 c)
c)
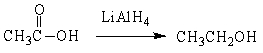
(Short Answer)
4.8/5  (40)
(40)
Consider the burning of methane gas (CH4):
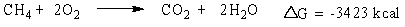 Which statement is true about the reaction?
Which statement is true about the reaction?
(Multiple Choice)
4.9/5  (35)
(35)
Figures A and B below represent reaction energy diagrams. Diagram that represents an endothermic reaction is 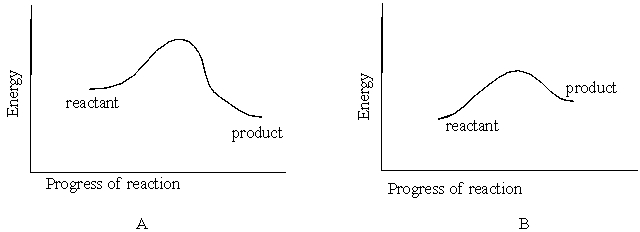
(Multiple Choice)
4.9/5  (37)
(37)
Classify the reaction below as involving synthesis, decomposition, single replacement, or double replacement. 
(Multiple Choice)
4.7/5  (36)
(36)
Classify the reaction below as involving synthesis, decomposition, single replacement, or double replacement. 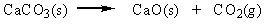
(Multiple Choice)
4.7/5  (38)
(38)
Isooctane, C8H18, a component of gasoline is a liquid at room temperature. How many moles of oxygen are required to completely react with 4 moles of isooctane? 
(Multiple Choice)
4.9/5  (47)
(47)
The percent yield of a reaction is determined by dividing ___ in grams or moles by the ___ in grams or moles times 100%
(Multiple Choice)
4.8/5  (36)
(36)
Which set of coefficients properly balance the equation below? 
(Multiple Choice)
4.8/5  (32)
(32)
Showing 61 - 80 of 90
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)