Exam 5: Reactions
Exam 1: Science and Measurements81 Questions
Exam 2: Atoms and Elements79 Questions
Exam 3: Compounds82 Questions
Exam 4: An Introduction to Organic Compounds75 Questions
Exam 5: Reactions90 Questions
Exam 6: Gases, Solutions, Colloids, and Suspensions104 Questions
Exam 7: Acids, Bases, and Equilibrium91 Questions
Exam 8: Organic Reactions Hydrocarbons, Carboxlic Acids, Amines, and Related Compounds77 Questions
Exam 9: Organic Reactions 2-Alcohols, Ethers, Aldehydes, and Ketones85 Questions
Exam 10: Carbohydrates84 Questions
Exam 11: Lipids and Membranes90 Questions
Exam 12: Peptides, Proteins, and Enzymes86 Questions
Exam 13: Nucleic Acids99 Questions
Exam 14: Metabolism84 Questions
Select questions type
What mass of potassium chloride, a salt substitute often used by heart patients, can be produced directly from 5.2 g potassium and 7.9 g chlorine? 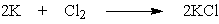
(Multiple Choice)
4.9/5  (34)
(34)
When properly balanced with whole number coefficients, what is the coefficient on H2O?
____HCl + ____Ba(OH)2 ___BaCl2 + ___H2O
(Multiple Choice)
4.7/5  (32)
(32)
If the charge on an ion changes from 2+ to 4+, the process occurring is ___.
(Short Answer)
4.8/5  (32)
(32)
Barium and sulfur react to produce barium sulfide. What is the role of barium in this reaction? 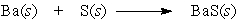
(Multiple Choice)
4.8/5  (44)
(44)
Tums tablets are composed of the substance calcium carbonate. Two tablets have a combined mass of 5.0 grams. Calcium carbonate reacts with and neutralizes excess stomach acid (hydrochloric acid). The products of this reaction are: water, calcium chloride, and carbon dioxide. How many moles of calcium carbonate are in one tablet?
(Short Answer)
4.7/5  (37)
(37)
A synthesis reaction in which water is added to a double bond is called ___.
(Short Answer)
4.9/5  (28)
(28)
What was the earliest reported method of fighting infection in the operating room?
(Multiple Choice)
4.9/5  (35)
(35)
Showing 81 - 90 of 90
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)