Exam 5: Reactions
Exam 1: Science and Measurements81 Questions
Exam 2: Atoms and Elements79 Questions
Exam 3: Compounds82 Questions
Exam 4: An Introduction to Organic Compounds75 Questions
Exam 5: Reactions90 Questions
Exam 6: Gases, Solutions, Colloids, and Suspensions104 Questions
Exam 7: Acids, Bases, and Equilibrium91 Questions
Exam 8: Organic Reactions Hydrocarbons, Carboxlic Acids, Amines, and Related Compounds77 Questions
Exam 9: Organic Reactions 2-Alcohols, Ethers, Aldehydes, and Ketones85 Questions
Exam 10: Carbohydrates84 Questions
Exam 11: Lipids and Membranes90 Questions
Exam 12: Peptides, Proteins, and Enzymes86 Questions
Exam 13: Nucleic Acids99 Questions
Exam 14: Metabolism84 Questions
Select questions type
If ethene (H2C=CH2) were to react with water in an acid solution (H+ present), the result would be which of the following?
(Multiple Choice)
4.8/5  (34)
(34)
Hydrolysis, hydration and dehydration reactions can take place in our cells with the aid of ____, which are proteins that catalyze reactions.
(Short Answer)
4.9/5  (32)
(32)
Two methanol molecules can react in the presence of sulfuric acid to produce dimethyl ether. Calculate the percent yield for this reaction that was run in a laboratory starting with 50 grams methanol and producing 26 grams dimethyl ether. 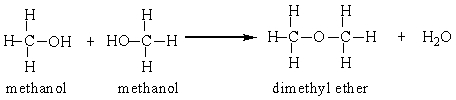
(Multiple Choice)
4.8/5  (34)
(34)
Aluminum carbonate was once the active ingredient in Rolaids . It reacts with stomach acid according to the following equation:
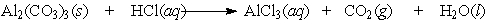
(Essay)
4.8/5  (36)
(36)
Ethene, C2H4, burns in air according to the equation below. What is the theoretical yield (in grams) of CO2 when 3.24 g of C2H4 are reacted with 4.83 g of O2? 
(Multiple Choice)
4.9/5  (32)
(32)
If 2.00 grams of potassium are allowed to react with 2.00 grams of bromine by the following equation, which reactant is the limiting reactant? 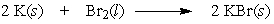
(Multiple Choice)
4.7/5  (38)
(38)
Balance the equation. Tums tablets are composed of the substance calcium carbonate. Two tablets have a combined mass of 5.0 grams. Calcium carbonate reacts with and neutralizes excess stomach acid (hydrochloric acid). The products of this reaction are: water, calcium chloride, and carbon dioxide.
(Essay)
4.9/5  (31)
(31)
The reaction below can be classified as a double replacement reaction.

(True/False)
4.9/5  (37)
(37)
What will you observe if you mix a solution of KOH with a solution of MgI2 in the reaction below? 
(Multiple Choice)
4.8/5  (39)
(39)
Classify the reaction below as involving synthesis, decomposition, single replacement, or double replacement. 
(Multiple Choice)
4.9/5  (39)
(39)
Pentose, C5H10O5, reacts with oxygen to produce carbon dioxide and water. What is the coefficient on O2 when the equation below is properly balanced? 
(Multiple Choice)
4.8/5  (25)
(25)
A chemist runs a reaction to prepare bromobenzene, C6H5Br, from the reaction of benzene (C6H6) with bromine(Br2):
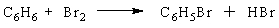 The theoretical yield of the reaction is 87 g of bromobenzene. If 68 g of bromobenzene are obtained, what is the percent yield of the reaction?
The theoretical yield of the reaction is 87 g of bromobenzene. If 68 g of bromobenzene are obtained, what is the percent yield of the reaction?
(Multiple Choice)
4.8/5  (30)
(30)
In a synthesis reaction, one compound breaks down to form elements or simpler compounds
(True/False)
4.9/5  (25)
(25)
Write the chemical equation for the reaction that takes place when trans-2-butene is mixed with H2 and Pt.
(Essay)
4.8/5  (31)
(31)
Baking soda (NaHCO3) can be used as an antacid. It reacts with and neutralizes stomach acid (HCl). The equation for this reaction is as follows:
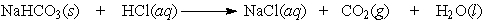 Calculate the mass (in grams) of hydrochloric acid that would be neutralized by 5.0 g of NaHCO3.
Calculate the mass (in grams) of hydrochloric acid that would be neutralized by 5.0 g of NaHCO3.
(Short Answer)
4.9/5  (33)
(33)
Showing 41 - 60 of 90
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)