Exam 13: Mixtures at the Molecular Level: Properties of Solutions
Exam 1: A Very Brief History of Chemistry90 Questions
Exam 2: Scientific Measurements224 Questions
Exam 3: Elements, Compounds, and the Periodic Table227 Questions
Exam 4: The Mole and Stoichiometry207 Questions
Exam 5: Molecular View of Reactions in Aqueous Solutions237 Questions
Exam 6: Oxidation-Reduction Reactions175 Questions
Exam 7: Energy and Chemical Change176 Questions
Exam 8: The Quantum Mechanical Atom219 Questions
Exam 9: The Basics of Chemical Bonding167 Questions
Exam 10: Theories of Bonding and Structure196 Questions
Exam 11: Properties of Gases162 Questions
Exam 12: Intermolecular Attractions and the Properties of Liquids and Solids189 Questions
Exam 13: Mixtures at the Molecular Level: Properties of Solutions133 Questions
Exam 14: Chemical Kinetics151 Questions
Exam 15: Chemical Equilibrium109 Questions
Exam 16: Acids and Bases, a Molecular Look104 Questions
Exam 17: Acid-Base Equilibria in Aqueous Solutions184 Questions
Exam 18: Solubility and Simultaneous Equilibria120 Questions
Exam 19: Thermodynamics109 Questions
Exam 20: Electrochemistry143 Questions
Exam 21: Nuclear Reactions and Their Role in Chemistry115 Questions
Exam 22: Metal Complexes113 Questions
Exam 23: Organic Compounds, Polymers, and Biochemicals140 Questions
Select questions type
After spilling vegetable oil on your clothes, you need to remove the stain. You have the following items available to help with the stain: vinegar (CH3CO2H); water (H2O); and toluene (C6H6). Which would be the best choice of solvent to remove the oil, and why?
(Short Answer)
4.8/5  (36)
(36)
A solution of potassium nitrate is prepared by mixing 3.50 g of KNO3 with 12.0 g of water. The percent, by mass, of KNO3 is
(Multiple Choice)
4.7/5  (30)
(30)
At 28.0°C, the vapor pressure of acetonitrile, CH3CN, is 102.0 torr while that of acetone, C3H6O, is 228.9 torr, and for CS2 the value is 378.7 torr. A three-component solution is made by adding 0.300 moles of CH3CN and 0.400 moles of C3H6O to 0.350 moles of CS2. The mixture behaves as an ideal mixture. What is the vapor pressure of the solution?Hint: Organizing the given information into moles and mole fraction of each component will help in solving this problem.
(Multiple Choice)
4.8/5  (38)
(38)
A molecular solute with a molar mass of 50.0 g mol-1 is dissolved in 500 g of water and the resulting solution has a boiling point of 101.53°C. How many grams of solute were in the solution Kb = 0.512 °C m-1
(Multiple Choice)
4.8/5  (39)
(39)
Consider a 0.80 M Al(NO3)3 solution. What would be the concentration of aluminum and nitrate ions in this solution? The aluminum concentration would be ________ M and the nitrate concentration would be ________ M.
(Short Answer)
4.7/5  (35)
(35)
Below is a diagram of vapor pressure versus composition for a mixture of two liquids. The dark straight lines represent the vapor pressure of each pure liquid, while the gray curved line gives the vapor pressure of the mixture. Using this information, which of the following statements is true about the mixture? 
(Multiple Choice)
4.8/5  (36)
(36)
Which response lists all the following pairs that are miscible liquids?
Pair 1. octane (C8H18)and water
Pair 2. ammonia (NH3)and water
Pair 3. octane (C8H18)and carbon tetrachloride (CCl4)
(Multiple Choice)
4.8/5  (38)
(38)
An aqueous solution of glycerol, C3H8O3, in which the mole fraction of C3H8O3 is 0.07070 was found to have a density of 1.0350 g mL-1. What is the molarity of this solution?Hint: Assume one mole of glycerol and solve for moles of water. From there you can find the mass and then volume of the solution.
(Multiple Choice)
4.9/5  (38)
(38)
Substances that dissolve more readily in oils than in water is best classified as ________.
(Short Answer)
4.7/5  (34)
(34)
A solution is prepared by mixing 0.3355 moles of NaNO3 (84.995 g mol-1)with 235.0 g of water (18.015 g mol-1). Its density is 1.0733 g mL-1. What is the molality of the solution?
(Multiple Choice)
4.9/5  (33)
(33)
An aqueous solution which is 12.00% sodium hydroxide by weight has a freezing point of ?10.40°C. What is the observed value of the van't Hoff factor, i, in this solution? Kf = 1.86 °C m-1.
(Multiple Choice)
4.8/5  (33)
(33)
At 23.0°C, the vapor pressure of acetonitrile, CH3CN, is 81.0 torr while that of acetone, C3H6O, is 184.5 torr. What is the vapor pressure of a solution that contains 0.550 moles of acetonitrile and 0.350 moles of acetone? (Assume the mixture behaves as an ideal solution.)
(Multiple Choice)
4.9/5  (35)
(35)
What is the osmotic pressure of a solution prepared from 13.7 g of HCl and water to make 0.500 L of solution at 18°C? Assume HCl completely ionizes.Hint: be sure to keep track of units when working this problem.
(Multiple Choice)
4.9/5  (35)
(35)
The Tyndall effect is a light effect that is observed with what kind of solution or mixture?
(Short Answer)
4.9/5  (37)
(37)
A solution of lithium hydroxide is prepared by mixing 2.00 g of LiOH with 10.0 g of water. The percent, by mass, of LiOH is
(Multiple Choice)
4.9/5  (38)
(38)
Pure benzene, C6H6, has a molar mass of 78.114 g mol−1, a density of 0.8765 g mL−1, a freezing point of 5.45°C, and a boiling point of 80.2°C. Its freezing point depression and boiling point elevation constants are: Kf = 5.07°C m−1; Kb = 2.53°C m−1. A solution was made by taking 33.88 g of an unknown nonelectrolyte and dissolving it in 175.0 g of benzene. The measured freezing point of the solution was -1.65°C. Calculate the molar mass of the unknown substance.Hint: Find molality, then moles, then molar mass, being sure to keep track of units.
(Short Answer)
4.9/5  (32)
(32)
A solution is prepared by mixing 0.3355 moles of NaNO3 with 235.0 g of water. Its density is 1.0733 g mL-1. If Kf = 1.86 °C m-1 and the solute is completely ionized, what is the expected freezing point of the solution?Hint: be sure to classify the solute as an electrolyte or non-electrolyte.
(Multiple Choice)
4.9/5  (34)
(34)
Below is a diagram of vapor pressure versus composition for a mixture of two liquids. The dark straight lines represent the vapor pressure of each pure liquid, while the gray curved line gives the vapor pressure of the mixture. Using this information, which of the following statements is true about the mixture? 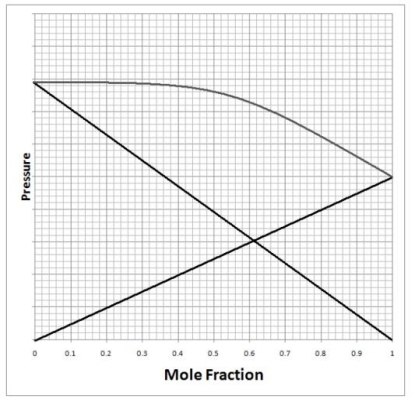
(Multiple Choice)
4.7/5  (37)
(37)
Showing 81 - 100 of 133
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)