Exam 13: Mixtures at the Molecular Level: Properties of Solutions
Exam 1: A Very Brief History of Chemistry90 Questions
Exam 2: Scientific Measurements224 Questions
Exam 3: Elements, Compounds, and the Periodic Table227 Questions
Exam 4: The Mole and Stoichiometry207 Questions
Exam 5: Molecular View of Reactions in Aqueous Solutions237 Questions
Exam 6: Oxidation-Reduction Reactions175 Questions
Exam 7: Energy and Chemical Change176 Questions
Exam 8: The Quantum Mechanical Atom219 Questions
Exam 9: The Basics of Chemical Bonding167 Questions
Exam 10: Theories of Bonding and Structure196 Questions
Exam 11: Properties of Gases162 Questions
Exam 12: Intermolecular Attractions and the Properties of Liquids and Solids189 Questions
Exam 13: Mixtures at the Molecular Level: Properties of Solutions133 Questions
Exam 14: Chemical Kinetics151 Questions
Exam 15: Chemical Equilibrium109 Questions
Exam 16: Acids and Bases, a Molecular Look104 Questions
Exam 17: Acid-Base Equilibria in Aqueous Solutions184 Questions
Exam 18: Solubility and Simultaneous Equilibria120 Questions
Exam 19: Thermodynamics109 Questions
Exam 20: Electrochemistry143 Questions
Exam 21: Nuclear Reactions and Their Role in Chemistry115 Questions
Exam 22: Metal Complexes113 Questions
Exam 23: Organic Compounds, Polymers, and Biochemicals140 Questions
Select questions type
The vapor pressure of a solution containing a nonvolatile solute is directly proportional to the
(Multiple Choice)
4.9/5  (33)
(33)
A phase diagram, like the one below, for an aqueous solution shows a shift in the solid/liquid and liquid/gas phase transitions but there is not a shift in the sublimation line between solids and gases. Explain what causes the shift in the other transitions and why this does not change a shift in the solid/gas transition.Hint: Think about the difference in vapor pressure between a solution and a pure substance. 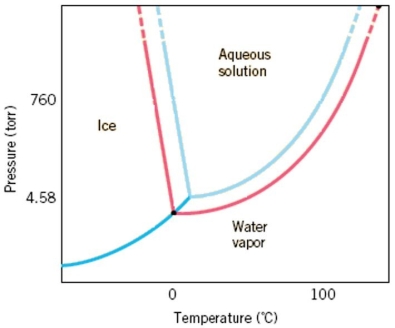
(Essay)
4.8/5  (41)
(41)
The CO2 gas sealed inside a carbonated beverage bottle has a pressure of 3.750 atm. At this pressure, the solubility of CO2 in water is 0.65 g CO2/100 g H2O. If the bottle is opened, as the gas in the space above the liquid escapes, the partial pressure of the CO2 falls to 0.30 torr, the value in the surrounding atmosphere of the room. What is the solubility of CO2 in the beverage at this new pressure?Hint: Be sure check your pressure units.
(Multiple Choice)
4.8/5  (30)
(30)
KBr has a lattice energy of -682 kJ/mol and a hydration energy of -657 kJ/mol. Using this information, what is the heat of solution, Hsol, for KBr?Hint: What happens to the crystal lattice when an ionic compound dissolves?
(Multiple Choice)
4.8/5  (33)
(33)
Which can be used to calculate the osmotic pressure of a solution?
(Multiple Choice)
4.9/5  (38)
(38)
Pure glacial acetic acid, HC2H3O2, has a freezing point of 16.62°C. Its freezing point depression constant is: Kf = 3.57 °C m-1. A solution was made by taking 9.755 g of an unknown nonelectrolyte and dissolving it in 90.50 g of glacial acetic acid. The measured freezing point of the solution was 8.64°C. Calculate the molecular weight of the unknown substance.Hint: Find molality, then moles, then molar mass, being sure to keep track of units.
(Multiple Choice)
4.8/5  (35)
(35)
Liquids that are mutually miscible possess intermolecular forces of similar type and magnitude.
(True/False)
4.9/5  (35)
(35)
A solution in a beaker has some undissolved solute lying on the bottom of the beaker. If the rate of crystallization exceeds the rate of dissolution of the excess solute, the solution is described as
(Multiple Choice)
4.7/5  (35)
(35)
A solution of chloroform (CHCl3)and acetone ((CH3)2CO)exhibits a negative deviation from Raoult's law similar to that shown on the diagram below. What does this tell us about the solution with respect to it being an ideal solution and the strength of the interactions between acetone and chloroform? This result shows us that the solution is ________ ideal and the interactions between chloroform-chloroform and acetone-acetone are ________ than the interactions between chloroform/acetone. 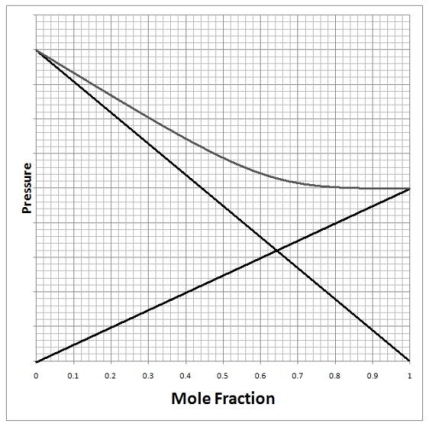
(Short Answer)
4.9/5  (47)
(47)
One driving force toward formation of homogeneous gas mixtures is the spontaneity of mixing through random motion of small molecules.
(True/False)
4.9/5  (32)
(32)
An aqueous solution of glycerol, C3H8O3 is prepared by adding 150.0 g of glycerol to 200.0 g of water. What is the mole fraction of glycerol in the final solution?
(Multiple Choice)
4.9/5  (32)
(32)
The solubility of O2 in water is approximately 0.00380 g L-1 when the temperature is 25.0°C and the partial pressure of gaseous oxygen is 760. torr. What will the solubility of oxygen be if the oxygen pressure is adjusted to 1000 torr?
(Multiple Choice)
4.8/5  (36)
(36)
Which aqueous solution will have the lowest boiling point temperature?
(Multiple Choice)
4.8/5  (28)
(28)
Which aqueous solution should have the highest osmotic pressure?
(Multiple Choice)
4.7/5  (30)
(30)
Pure benzene, C6H6, has a molar mass of 78.114 g mol−1, a density of 0.8765 g mL−1, a freezing point of 5.45°C, and a boiling point of 80.2°C. Its freezing point depression and boiling point elevation constants are: Kf = 5.07 °C m−1; Kb = 2.53 °C m−1. A solution was made by taking 16.00 g of an unknown nonelectrolyte and dissolving it in 168.0 g of benzene. The measured freezing point of the solution was -1.05°C. Calculate the molar mass of the unknown substance.Hint: Find molality, then moles, then molar mass, being sure to keep track of units.
(Short Answer)
4.7/5  (32)
(32)
How many grams of water are needed to dissolve 13.9 g of ammonium nitrate NH4NO3 in order to prepare a 0.452 m solution?
(Short Answer)
4.8/5  (34)
(34)
A solution is prepared by mixing 0.3355 moles of NaNO3 (84.995 g mol-1)with 235.0 g of water (18.015 g mol-1). Its density is 1.0733 g mL-1. What is the percent by mass of NaNO3 in the solution?
(Multiple Choice)
4.9/5  (38)
(38)
What is the normal boiling point of a solution that contains 30.0 g of glucose (C6H12O6)in 100.0 g of H2O? Kb for water is 0.512°C/m.
(Multiple Choice)
4.8/5  (34)
(34)
Pure cyclohexane, C6H12, has a molar mass of 84.161 g mol−1, a density of 0.7785 g mL−1, a freezing point of 6.53°C, and a boiling point of 80.72°C. Its freezing point depression and boiling point elevation constants are: Kf = 20.0 °C m−1; Kb = 2.69 °C m−1. A solution was made by taking 9.50 g of an unknown nonelectrolyte and dissolving it in 125.0 g of cyclohexane. The measured freezing point of the solution was -0.78°C. Calculate the molar mass of the unknown substance.Hint: Find molality, then moles, then molar mass, being sure to keep track of units.
(Short Answer)
4.7/5  (30)
(30)
Raoult's law expresses that the higher the mole fraction of a volatile solvent in an ideal solution the ________ its partial pressure above the solution.
(Short Answer)
4.8/5  (33)
(33)
Showing 21 - 40 of 133
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)