Exam 13: Mixtures at the Molecular Level: Properties of Solutions
Exam 1: A Very Brief History of Chemistry90 Questions
Exam 2: Scientific Measurements224 Questions
Exam 3: Elements, Compounds, and the Periodic Table227 Questions
Exam 4: The Mole and Stoichiometry207 Questions
Exam 5: Molecular View of Reactions in Aqueous Solutions237 Questions
Exam 6: Oxidation-Reduction Reactions175 Questions
Exam 7: Energy and Chemical Change176 Questions
Exam 8: The Quantum Mechanical Atom219 Questions
Exam 9: The Basics of Chemical Bonding167 Questions
Exam 10: Theories of Bonding and Structure196 Questions
Exam 11: Properties of Gases162 Questions
Exam 12: Intermolecular Attractions and the Properties of Liquids and Solids189 Questions
Exam 13: Mixtures at the Molecular Level: Properties of Solutions133 Questions
Exam 14: Chemical Kinetics151 Questions
Exam 15: Chemical Equilibrium109 Questions
Exam 16: Acids and Bases, a Molecular Look104 Questions
Exam 17: Acid-Base Equilibria in Aqueous Solutions184 Questions
Exam 18: Solubility and Simultaneous Equilibria120 Questions
Exam 19: Thermodynamics109 Questions
Exam 20: Electrochemistry143 Questions
Exam 21: Nuclear Reactions and Their Role in Chemistry115 Questions
Exam 22: Metal Complexes113 Questions
Exam 23: Organic Compounds, Polymers, and Biochemicals140 Questions
Select questions type
The solubility of O2 in water is approximately 0.00380 g L-1 of water when the temperature is 25.0°C and the partial pressure of gaseous oxygen is 760 torr. The oxygen gas above the water is replaced by air at the same temperature and pressure, in which the mole fraction of oxygen is 0.210. What will the solubility of oxygen in water be under these new conditions?
(Multiple Choice)
5.0/5  (31)
(31)
An aqueous solution of glycerol, C3H8O3, is 48.0% glycerol by mass andhas a density of 1.120 g mL-1. Calculate the molarity of the glycerol solution.
(Multiple Choice)
4.8/5  (35)
(35)
A solution of ethylene glycol (C2H6O2)in water is 3.981 molar and has a density of 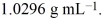 Calculate the percent, by weight, of ethylene glycol in the solution.
Calculate the percent, by weight, of ethylene glycol in the solution.
(Multiple Choice)
4.9/5  (31)
(31)
An aqueous solution that would cause red blood cells to shrink from increased osmotic pressure is called a(n)________ solution.
(Multiple Choice)
4.8/5  (36)
(36)
If the concentration of hydrogen gas in a liquid sample at 1.25 atm and 25°C is 9.75 × 10−4 M, the concentration of hydrogen gas at 3.41 atm and 25°C would be ________.
(Short Answer)
4.9/5  (26)
(26)
All substances can be either hydrophobic or hydrophilic, but not both.
(True/False)
4.7/5  (42)
(42)
An aqueous solution of glycerol, C3H8O3, is 48.0% glycerol by mass and has a density of 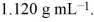 Calculate the molality of the glycerol solution.
Calculate the molality of the glycerol solution.
(Multiple Choice)
4.9/5  (37)
(37)
Pure cyclohexane, C6H12, has a freezing point of 6.53°C. Its freezing point depression constant is: Kf = 20.0 °C m-1. A solution was made by taking 11.40 g of an unknown nonelectrolyte and dissolving it in 150.0 g of cyclohexane. The measured freezing point of the solution was -0.78°C. Calculate the molecular weight of the unknown substance.Hint: Find molality, then moles, then molar mass, being sure to keep track of units.
(Multiple Choice)
4.8/5  (31)
(31)
Which is a concentration unit whose value changes if the temperature of an aqueous solution is changed?
(Multiple Choice)
4.8/5  (43)
(43)
What is the freezing point of an aqueous solution of a nonvolatile solute that has a boiling point of 102.45 °C? Kf = 1.86 °C m−1 and Kb = 0.512 °C m−1.
(Short Answer)
4.9/5  (44)
(44)
A solution contains 221 g of glycerol (C3H8O3)in 600 grams of water. For the solvent, the Kf is 1.86 °C m-1 and Kb is 0.512 °C m-1. What should the boiling point of the solution be?
(Multiple Choice)
4.9/5  (39)
(39)
A very dilute solution contains 116 mg of fructose (molar mass = 180.16 g mol-1)in 1.000 liter of solution. It is placed in an osmotic membrane bladder, which is then suspended in pure water. What osmotic pressure would develop across the membrane if the temperature is 26.0°C?
(Multiple Choice)
5.0/5  (34)
(34)
The solubility of CO2 gas in water would ________ if the partial pressure of CO2 was increased.
(Short Answer)
4.8/5  (38)
(38)
A solution, which was made by dissolving 62.07 g of a nonelectrolyte in 500 g of water, exhibits a freezing point of -1.86°C. What is the molecular weight of this nonelectrolyte compound? For water, Kf is 1.86°C m-1 and Kb is 0.512°C m-1.
(Multiple Choice)
4.8/5  (43)
(43)
An aqueous solution of ethanol, C2H5OH is prepared by adding 200. g of ethanol to 50.0 g of water. What is the mole fraction of ethanol in the final solution?
(Multiple Choice)
4.8/5  (34)
(34)
The solubility of oxygen in lakes high in mountains is affected by the altitude. If the solubility of O2 from the air is 2.67 × 10−4 M at sea level when the pressure is 1.000 atm and the temperature is 25°C, what is the solubility of O2 at an elevation of 12,000 ft. where the atmospheric pressure is 0.643 atm? Assume the temperature is 25°C, and that the mole fraction of O2 in air is 0.211 at both 12,000 ft. and at sea level.
(Short Answer)
4.9/5  (41)
(41)
Colligative properties depend primarily on the concentration of solute particles in the solution.
(True/False)
4.9/5  (32)
(32)
Showing 41 - 60 of 133
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)