Exam 12: Intermolecular Attractions and the Properties of Liquids and Solids
Exam 1: A Very Brief History of Chemistry90 Questions
Exam 2: Scientific Measurements224 Questions
Exam 3: Elements, Compounds, and the Periodic Table227 Questions
Exam 4: The Mole and Stoichiometry207 Questions
Exam 5: Molecular View of Reactions in Aqueous Solutions237 Questions
Exam 6: Oxidation-Reduction Reactions175 Questions
Exam 7: Energy and Chemical Change176 Questions
Exam 8: The Quantum Mechanical Atom219 Questions
Exam 9: The Basics of Chemical Bonding167 Questions
Exam 10: Theories of Bonding and Structure196 Questions
Exam 11: Properties of Gases162 Questions
Exam 12: Intermolecular Attractions and the Properties of Liquids and Solids189 Questions
Exam 13: Mixtures at the Molecular Level: Properties of Solutions133 Questions
Exam 14: Chemical Kinetics151 Questions
Exam 15: Chemical Equilibrium109 Questions
Exam 16: Acids and Bases, a Molecular Look104 Questions
Exam 17: Acid-Base Equilibria in Aqueous Solutions184 Questions
Exam 18: Solubility and Simultaneous Equilibria120 Questions
Exam 19: Thermodynamics109 Questions
Exam 20: Electrochemistry143 Questions
Exam 21: Nuclear Reactions and Their Role in Chemistry115 Questions
Exam 22: Metal Complexes113 Questions
Exam 23: Organic Compounds, Polymers, and Biochemicals140 Questions
Select questions type
The presence of which intermolecular force would lead to the highest viscosity within a liquid?
(Multiple Choice)
4.9/5  (42)
(42)
During a rain storm, the surface of a car hood gets wet. If the car has a good polish or has recently been waxed, the water tends to bead or form drops, instead of evenly covering the surface. What does this say about the properties of water and the wax used to protect the car hood?
(Short Answer)
4.7/5  (39)
(39)
What is the mass of a sample of iron that has 5.00 × 1022 unit cells, if the iron crystallizes in a body-centered cubic lattice? The molar mass of iron is 55.85 g mol-1.Hint: Use dimensional analysis and the number of atoms per unit cell.
(Multiple Choice)
4.7/5  (30)
(30)
A low heat of vaporization indicates that a substance has ________ intermolecular forces in the liquid form.
(Short Answer)
4.8/5  (36)
(36)
An unknown solid is very hard with a very high melting point. It does not conduct electricity in either the solid or liquid phase. What kind of crystal best describes this solid?
(Multiple Choice)
4.9/5  (28)
(28)
Which of the following organic liquids would have the strongest surface tension?
(Multiple Choice)
4.8/5  (37)
(37)
Which of the following compounds should have the largest value for heat of vaporization ( Hvap)?
(Multiple Choice)
4.8/5  (40)
(40)
A liquid which is kept in a pressure container at a temperature above its critical temperature is called a ________.
(Short Answer)
4.9/5  (40)
(40)
The following questions refer to the diagram below. 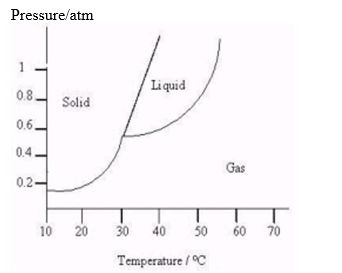 -At what temperature and pressure will the species stop subliming (going from a solid to a gas)?
-At what temperature and pressure will the species stop subliming (going from a solid to a gas)?
(Short Answer)
4.9/5  (40)
(40)
Boron nitride has the empirical formula BN, melts at 2967°C and is nearly as hard as diamond. Which category of substance does boron nitride most likely fit into?
(Multiple Choice)
4.8/5  (37)
(37)
The normal boiling point of acetic acid, HC2H3O2, is 117.9°C, and its heat of vaporization is 39,690 J/mol. What is its vapor pressure at 100.0°C?
(Multiple Choice)
4.9/5  (36)
(36)
An unknown solid is soft and brittle with a low melting point. It does not conduct electricity in either the solid or liquid phase. What kind of crystal best describes this solid?
(Multiple Choice)
4.9/5  (35)
(35)
X-ray diffraction measurements on a newly discovered crystalline form of element X revealed that X crystallizes in a body-centered cubic lattice in which the unit cell edge length is 304.6 picometers. Calculate the atomic radius of the X atoms in this crystal, based on the assumption that the atoms are tightly packed in the unit cell.Hint: Find the length of the cell body diagonal using the Pythagorean theorem.
(Multiple Choice)
4.8/5  (39)
(39)
X-ray diffraction measurements on a newly discovered crystalline form of Eu revealed that Eu crystallizes in a body-centered cubic lattice in which the unit cell edge length is 458.3 picometers. Calculate the atomic radius of the Eu atoms in this crystal, based on the assumption that the atoms are tightly packed in the unit cell.Hint: Find the length of the cell body diagonal using the Pythagorean theorem.
(Multiple Choice)
4.8/5  (36)
(36)
In order to boil water, we need to supply heat. This is in order to
(Multiple Choice)
4.9/5  (46)
(46)
Describe the difference between molecular crystals and covalent crystals, in terms of types of bonding and physical properties.
(Essay)
4.8/5  (33)
(33)
What compound will not exhibit hydrogen bonding in the liquid state?
(Multiple Choice)
4.7/5  (44)
(44)
X-ray diffraction measurements revealed that Ni crystallizes in a face-centered cubic lattice in which the unit cell edge length is 352.4 picometers.Calculate the atomic radius of the Ni atoms in this crystal, based on the assumption that the atoms are tightly packed in the unit cell.Hint: Find the length of the cell face diagonal using the Pythagorean theorem.
(Multiple Choice)
4.8/5  (37)
(37)
What other molecular factors determine the viscosity of a liquid?
(Short Answer)
4.9/5  (34)
(34)
Showing 81 - 100 of 189
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)