Exam 12: Intermolecular Attractions and the Properties of Liquids and Solids
Exam 1: A Very Brief History of Chemistry90 Questions
Exam 2: Scientific Measurements224 Questions
Exam 3: Elements, Compounds, and the Periodic Table227 Questions
Exam 4: The Mole and Stoichiometry207 Questions
Exam 5: Molecular View of Reactions in Aqueous Solutions237 Questions
Exam 6: Oxidation-Reduction Reactions175 Questions
Exam 7: Energy and Chemical Change176 Questions
Exam 8: The Quantum Mechanical Atom219 Questions
Exam 9: The Basics of Chemical Bonding167 Questions
Exam 10: Theories of Bonding and Structure196 Questions
Exam 11: Properties of Gases162 Questions
Exam 12: Intermolecular Attractions and the Properties of Liquids and Solids189 Questions
Exam 13: Mixtures at the Molecular Level: Properties of Solutions133 Questions
Exam 14: Chemical Kinetics151 Questions
Exam 15: Chemical Equilibrium109 Questions
Exam 16: Acids and Bases, a Molecular Look104 Questions
Exam 17: Acid-Base Equilibria in Aqueous Solutions184 Questions
Exam 18: Solubility and Simultaneous Equilibria120 Questions
Exam 19: Thermodynamics109 Questions
Exam 20: Electrochemistry143 Questions
Exam 21: Nuclear Reactions and Their Role in Chemistry115 Questions
Exam 22: Metal Complexes113 Questions
Exam 23: Organic Compounds, Polymers, and Biochemicals140 Questions
Select questions type
Silicon carbide, which has the empirical formula SiC, melts at 2830°C and is nearly as hard as diamond. Which category of substance does silicon carbide most likely fit into?
(Multiple Choice)
5.0/5  (44)
(44)
Which type of unit cell has lattice points at each corner, and one in the center of the cell?
(Multiple Choice)
4.9/5  (33)
(33)
Osmium tetroxide, OsO4, forms a crystal that melts at 40°C. The liquid formed from this melting does not conduct electricity. What would be the best classification of this type of crystal?
(Short Answer)
4.9/5  (45)
(45)
Based on the phase diagram of water, why can someone on ice skates can glide along the ice much more easily than someone in normal shoes?
(Short Answer)
4.9/5  (42)
(42)
Determine the shift in equilibrium that would result if 100 kJ of energy were added to water freezing at 0°C to form ice (H2O(s)⇆ H2O(l)).
(Short Answer)
4.9/5  (35)
(35)
Which molecule is most polarizable and subject to the greatest instantaneous dipole-induced dipole forces?
(Multiple Choice)
4.8/5  (43)
(43)
Which of the following liquids, at the same temperature, has the highest vapor pressure?
(Multiple Choice)
4.9/5  (42)
(42)
X-ray diffraction measurements revealed that Ag crystallizes in a face-centered cubic lattice in which the unit cell edge length is 408.6 picometers. Calculate the atomic radius of the Ag atoms in this crystal, based on the usual assumption that the atoms are tightly packed in the unit cell.
(Multiple Choice)
4.8/5  (39)
(39)
A high vapor pressure indicates that the substance has ________ intermolecular forces in the liquid form.
(Short Answer)
4.9/5  (28)
(28)
The vapor pressure of a liquid increases with increasing temperature. The temperature at which the vapor pressure is equal to the prevailing outside atmospheric pressure is
(Multiple Choice)
4.8/5  (30)
(30)
Draw the unit cell for the following two-dimensional lattice. 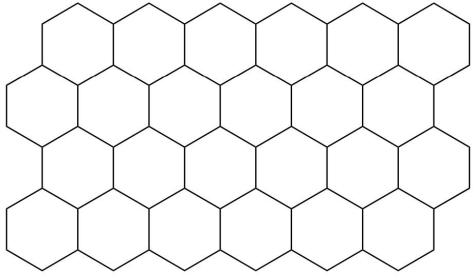 Hint: Think about the repeating unit.
Hint: Think about the repeating unit.
(Short Answer)
4.9/5  (43)
(43)
The pressure below which the liquid state of matter does not exist regardless of the temperature is called ________.
(Short Answer)
4.8/5  (43)
(43)
Which factor affects the normal boiling point of a liquid?
(Multiple Choice)
4.8/5  (41)
(41)
Determine the shift in equilibrium that would result if 100 kJ of energy were removed from a system in which dry ice and CO2 gas were in equilibrium at the freezing point.
(Short Answer)
4.9/5  (37)
(37)
Which type of lattice structures have lattice points on all of the faces?
(Multiple Choice)
4.8/5  (35)
(35)
An unknown solid is hard, but brittle and melts at 3500°C. The liquid form of the unknown conducts electricity, but the solid form does not. What kind of crystal is the unknown?
(Short Answer)
4.9/5  (38)
(38)
Substance A has a normal melting point of -25.0°C, a heat of fusion of 1200 J g-1; specific heats for the solid and the liquid are 3.00 and 6.20 J g-1 °C -1, respectively. How much heat will be required to change 150 grams of substance A from a solid at -40.0°C to a liquid at +70.0°C?
(Multiple Choice)
4.8/5  (42)
(42)
An unknown solid is hard, but malleable and melts at 1700°C. The liquid form conducts electricity. The solid form also conducts electricity. What kind of crystal is this?
(Short Answer)
5.0/5  (36)
(36)
The unit cell below is best described as what type of crystal structure? 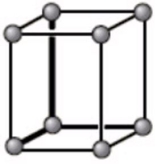
(Multiple Choice)
4.9/5  (27)
(27)
Showing 161 - 180 of 189
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)