Exam 12: Intermolecular Attractions and the Properties of Liquids and Solids
Exam 1: A Very Brief History of Chemistry90 Questions
Exam 2: Scientific Measurements224 Questions
Exam 3: Elements, Compounds, and the Periodic Table227 Questions
Exam 4: The Mole and Stoichiometry207 Questions
Exam 5: Molecular View of Reactions in Aqueous Solutions237 Questions
Exam 6: Oxidation-Reduction Reactions175 Questions
Exam 7: Energy and Chemical Change176 Questions
Exam 8: The Quantum Mechanical Atom219 Questions
Exam 9: The Basics of Chemical Bonding167 Questions
Exam 10: Theories of Bonding and Structure196 Questions
Exam 11: Properties of Gases162 Questions
Exam 12: Intermolecular Attractions and the Properties of Liquids and Solids189 Questions
Exam 13: Mixtures at the Molecular Level: Properties of Solutions133 Questions
Exam 14: Chemical Kinetics151 Questions
Exam 15: Chemical Equilibrium109 Questions
Exam 16: Acids and Bases, a Molecular Look104 Questions
Exam 17: Acid-Base Equilibria in Aqueous Solutions184 Questions
Exam 18: Solubility and Simultaneous Equilibria120 Questions
Exam 19: Thermodynamics109 Questions
Exam 20: Electrochemistry143 Questions
Exam 21: Nuclear Reactions and Their Role in Chemistry115 Questions
Exam 22: Metal Complexes113 Questions
Exam 23: Organic Compounds, Polymers, and Biochemicals140 Questions
Select questions type
What type of crystal lattice is found in a sample of table salt, NaCl?
(Multiple Choice)
4.7/5  (30)
(30)
Gold crystallizes in a face-centered cubic lattice. How many unit cells are there in a gold sample whose mass is 1.50 grams? The atomic weight of gold is 196.97 g mol-1.Hint: Use dimensional analysis and the number of atoms per unit cell.
(Multiple Choice)
4.9/5  (39)
(39)
X-ray diffraction measurements revealed that -Fe crystallizes in a face-centered cubic lattice in which the unit cell edge length is 286.6 picometers. Calculate the atomic radius of the Fe atoms in this crystal, based on the assumption that the atoms are tightly packed in the unit cell.Hint: Find the length of the cell face diagonal using the Pythagorean theorem.
(Multiple Choice)
4.7/5  (38)
(38)
When a solid undergoes a change of state to a gas, the process is called
(Multiple Choice)
4.8/5  (44)
(44)
Which compound will have the strongest intermolecular forces?
(Multiple Choice)
4.9/5  (31)
(31)
Which molecule is most polarizable and subject to significant instantaneous dipole-induced dipole forces?
(Multiple Choice)
4.8/5  (38)
(38)
A liquid will evaporate at a given temperature until ________ is reached.
(Short Answer)
4.9/5  (32)
(32)
The following questions refer to the basic phase diagram below with gas, liquid, and solid phases present and labeled. 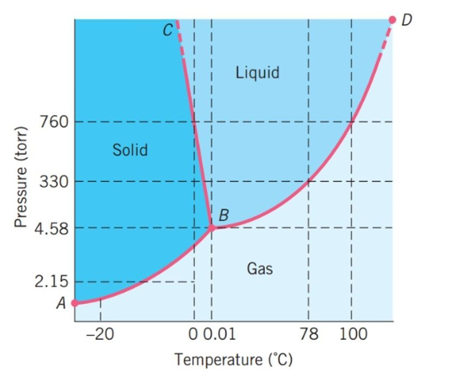 -If the substance starts at a pressure of 500 torr and 105°C and is cooled to 50°C, what phase transition will occur?
-If the substance starts at a pressure of 500 torr and 105°C and is cooled to 50°C, what phase transition will occur?
(Short Answer)
4.7/5  (48)
(48)
Which of the following is not a factor that directly affects the viscosity of a liquid?
(Multiple Choice)
4.8/5  (29)
(29)
Find the boiling temperature at 760 torr of an isomer of octane, C8H18, if its heat of vaporization is 38,210 J mol-1 and its vapor pressure at 110.0°C is 638.43 torr.
(Multiple Choice)
4.8/5  (37)
(37)
Iron crystallizes in a body-centered cubic lattice. How many unit cells are in an iron sample whose mass is 1.50 grams? The molar mass of iron is 55.85 g mol-1.Hint: Use dimensional analysis and the number of atoms per unit cell.
(Multiple Choice)
4.7/5  (33)
(33)
Using Le Châtelier's Principle and what you know about phase transitions, explain how a person coming out of a swimming pool in Arizona on a 90°F day is more likely to become cold than someone getting out of a swimming pool in Florida on a 90°F day.
(Short Answer)
4.9/5  (42)
(42)
22.4 g of a solid with a molecular weight of 148.0 g/mol is at its melting point. If this solid requires 5.358 kJ of heat to melt it, what is the molar heat of fusion, in kJ/mol, for this solid?
(Multiple Choice)
4.9/5  (35)
(35)
Which covalent compound will exhibit hydrogen bonding in the liquid state?
(Multiple Choice)
4.9/5  (30)
(30)
A substance with a high boiling point has ________ intermolecular forces in the liquid form.
(Short Answer)
4.7/5  (34)
(34)
The unit cell below is best described as what type of crystal structure? 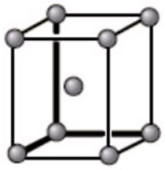
(Multiple Choice)
4.8/5  (36)
(36)
The ease with which the electron cloud of an atom or molecule is distorted is called the ________ of the atom or molecule.
(Short Answer)
4.9/5  (28)
(28)
The strongest intermolecular forces between molecules of NO are
(Multiple Choice)
4.9/5  (36)
(36)
The following questions refer to the phase diagram below. 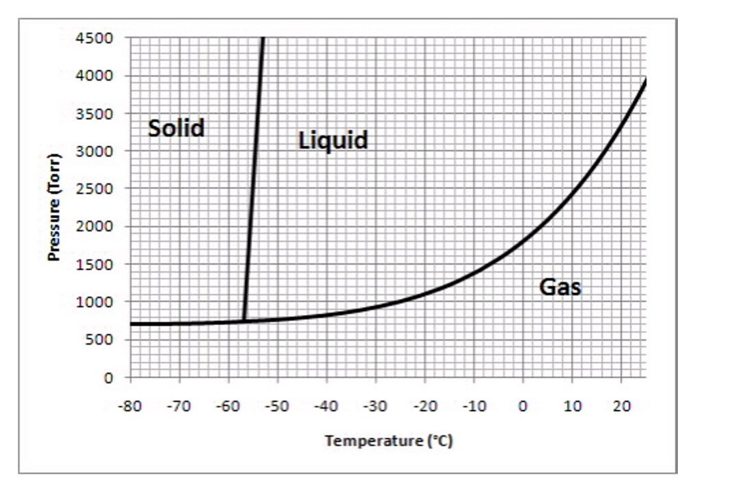 -What phase should this substance exist as, at a pressure of 1500 torr and a temperature of -20°C?
-What phase should this substance exist as, at a pressure of 1500 torr and a temperature of -20°C?
(Multiple Choice)
4.8/5  (48)
(48)
What lies in the geometric center of a unit cell of sodium chloride (face-centered cubic)?
(Multiple Choice)
4.8/5  (39)
(39)
Showing 141 - 160 of 189
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)