Exam 12: Intermolecular Attractions and the Properties of Liquids and Solids
Exam 1: A Very Brief History of Chemistry90 Questions
Exam 2: Scientific Measurements224 Questions
Exam 3: Elements, Compounds, and the Periodic Table227 Questions
Exam 4: The Mole and Stoichiometry207 Questions
Exam 5: Molecular View of Reactions in Aqueous Solutions237 Questions
Exam 6: Oxidation-Reduction Reactions175 Questions
Exam 7: Energy and Chemical Change176 Questions
Exam 8: The Quantum Mechanical Atom219 Questions
Exam 9: The Basics of Chemical Bonding167 Questions
Exam 10: Theories of Bonding and Structure196 Questions
Exam 11: Properties of Gases162 Questions
Exam 12: Intermolecular Attractions and the Properties of Liquids and Solids189 Questions
Exam 13: Mixtures at the Molecular Level: Properties of Solutions133 Questions
Exam 14: Chemical Kinetics151 Questions
Exam 15: Chemical Equilibrium109 Questions
Exam 16: Acids and Bases, a Molecular Look104 Questions
Exam 17: Acid-Base Equilibria in Aqueous Solutions184 Questions
Exam 18: Solubility and Simultaneous Equilibria120 Questions
Exam 19: Thermodynamics109 Questions
Exam 20: Electrochemistry143 Questions
Exam 21: Nuclear Reactions and Their Role in Chemistry115 Questions
Exam 22: Metal Complexes113 Questions
Exam 23: Organic Compounds, Polymers, and Biochemicals140 Questions
Select questions type
What is the difference between intermolecular interactions and intramolecular interactions in a molecule?
(Short Answer)
4.8/5  (38)
(38)
What is the mass of a sample of silver that has 5.00 × 1022 unit cells, if the silver crystallizes in a face-centered cubic lattice? The molar mass of silver is 107.87 g mol-1Hint: Use dimensional analysis and the number of atoms per unit cell.
(Multiple Choice)
4.9/5  (30)
(30)
Arrange these compounds in order of increasing intermolecular forces:CCl4, GeCl4, SiCl4, SnCl4.
(Short Answer)
4.9/5  (31)
(31)
The following questions refer to the phase diagram below. Gas, liquid, and solid phases are all present, but not labeled. 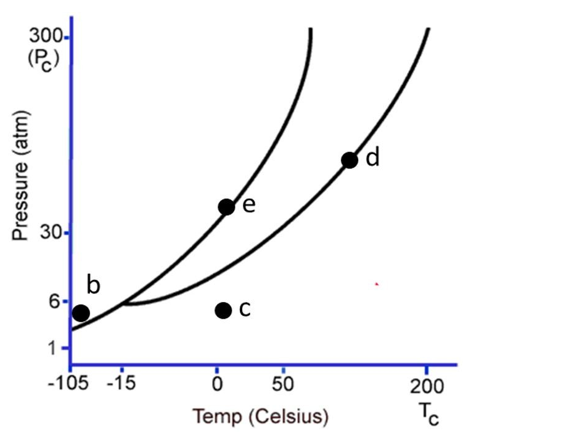 -Starting at the temperature and pressure of c, if the pressure is increased at constant temperature, ultimately
-Starting at the temperature and pressure of c, if the pressure is increased at constant temperature, ultimately
(Multiple Choice)
4.9/5  (40)
(40)
The following questions refer to the basic phase diagram below with gas, liquid, and solid phases present and labeled. 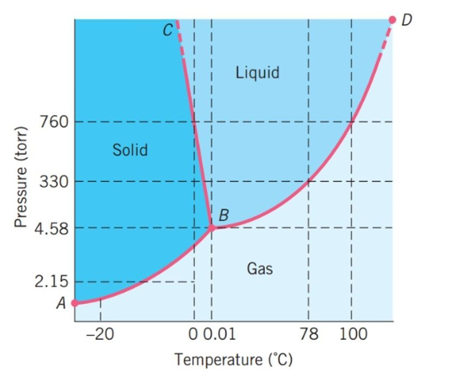 -What phase of this substance exists at 760 torr and 50°C?
-What phase of this substance exists at 760 torr and 50°C?
(Short Answer)
4.9/5  (39)
(39)
The heat capacity of liquid water is 4.18 J g-1 °C-1 and its heat of vaporization is 40.7 kJ/mol. From this information, ________ kJ of heat must be provided to convert 1.50 g of liquid water at 57°C into 1.50 g of steam at 100°C.
(Short Answer)
4.8/5  (34)
(34)
How many atoms are contained in one unit cell of metallic tungsten, if it forms a face-centered cubic unit cell?
(Multiple Choice)
4.8/5  (38)
(38)
Which property of water allows some bugs to be able to walk on the surface of water?
(Short Answer)
4.7/5  (42)
(42)
The surface tension of a liquid depends on the strength of the substance's intermolecular attractive forces.
(True/False)
4.7/5  (25)
(25)
A liquid in a covered container is in equilibrium with its vapor. If the cover is removed, what is the immediate result?
(Multiple Choice)
4.9/5  (33)
(33)
Given the following substances and their normal boiling points, in °C: 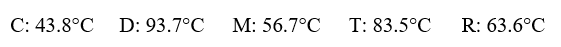 Which ranking correctly lists some of these substances in order of decreasing vapor pressure at 20°C?
Which ranking correctly lists some of these substances in order of decreasing vapor pressure at 20°C?
(Multiple Choice)
4.9/5  (32)
(32)
X-ray diffraction measurements revealed that Cu crystallizes in a face-centered cubic lattice in which the unit cell edge length is 361.5 picometers. Calculate the atomic radius of the Cu atoms in this crystal, based on the assumption that the atoms are tightly packed in the unit cell.Hint: Find the length of the cell face diagonal using the Pythagorean theorem.
(Multiple Choice)
4.8/5  (40)
(40)
Broken laboratory glass can sometimes be repaired by using heat to melt the glass pieces back together at the break or to melt shut a crack. Often the glass will crack easily at these repair joints, unless it undergoes a process called annealing. Annealing raises the temperature of the glass to just below the melting point and slowly lets it cool down to room temperature. After annealing, the glassware is often just like new. Using your knowledge of amorphous solids, why does annealing strengthen the glassware?
(Short Answer)
4.9/5  (31)
(31)
Benzophenone, C13H10O, has a heat of fusion of 98.45 J g-1 and its melting point temperature is 47.85°C. How much heat is required to transform 126 g of solid benzophenone at 47.85°C into liquid benzophenone, also at 47.85°C?
(Multiple Choice)
4.8/5  (22)
(22)
Which of the following compounds should have the largest value for heat of fusion ( Hfus)?
(Multiple Choice)
4.7/5  (39)
(39)
An isobar is a line of constant pressure which runs parallel to the temperature axis on the phase diagram for a substance. As we vary temperature along an isobar that lies below the triple point, which process would never be observed?
(Multiple Choice)
4.9/5  (35)
(35)
The following questions refer to the basic phase diagram below with gas, liquid, and solid phases present and labeled.  -The triple point of this substance is at ________ atm and ________ °C.
-The triple point of this substance is at ________ atm and ________ °C.
(Short Answer)
4.9/5  (36)
(36)
The unit cell below is best described as what type of crystal structure? 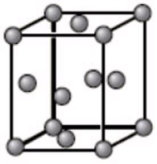
(Multiple Choice)
4.7/5  (42)
(42)
Showing 101 - 120 of 189
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)