Exam 12: Intermolecular Attractions and the Properties of Liquids and Solids
Exam 1: A Very Brief History of Chemistry90 Questions
Exam 2: Scientific Measurements224 Questions
Exam 3: Elements, Compounds, and the Periodic Table227 Questions
Exam 4: The Mole and Stoichiometry207 Questions
Exam 5: Molecular View of Reactions in Aqueous Solutions237 Questions
Exam 6: Oxidation-Reduction Reactions175 Questions
Exam 7: Energy and Chemical Change176 Questions
Exam 8: The Quantum Mechanical Atom219 Questions
Exam 9: The Basics of Chemical Bonding167 Questions
Exam 10: Theories of Bonding and Structure196 Questions
Exam 11: Properties of Gases162 Questions
Exam 12: Intermolecular Attractions and the Properties of Liquids and Solids189 Questions
Exam 13: Mixtures at the Molecular Level: Properties of Solutions133 Questions
Exam 14: Chemical Kinetics151 Questions
Exam 15: Chemical Equilibrium109 Questions
Exam 16: Acids and Bases, a Molecular Look104 Questions
Exam 17: Acid-Base Equilibria in Aqueous Solutions184 Questions
Exam 18: Solubility and Simultaneous Equilibria120 Questions
Exam 19: Thermodynamics109 Questions
Exam 20: Electrochemistry143 Questions
Exam 21: Nuclear Reactions and Their Role in Chemistry115 Questions
Exam 22: Metal Complexes113 Questions
Exam 23: Organic Compounds, Polymers, and Biochemicals140 Questions
Select questions type
The vapor pressure of ethanol is 400 mmHg at 63.5°C. Its molar heat of vaporization is 39.3 kJ/mol. What is the vapor pressure of ethanol at 34.9°C?
(Short Answer)
4.9/5  (35)
(35)
A cylinder contains a liquid which is in equilibrium with its vapor at 45.0°C. If the piston is moved down to constrict the volume of the cylinder without changing the temperature, the equilibrium vapor pressure of the liquid will increase.
(True/False)
4.8/5  (35)
(35)
Will a nonpolar molecule will have the strongest intermolecular interaction with another nonpolar molecule, a polar molecule, or an ion in solution? Explain.
(Essay)
4.8/5  (47)
(47)
Which compound will have the weakest intermolecular forces?
(Multiple Choice)
4.7/5  (39)
(39)
Arrange these compounds in order of increasing vapor pressure at 20 °C:CBr4, CCl4, CF4, CH4, CI4.
(Short Answer)
4.9/5  (34)
(34)
The following questions refer to the phase diagram below. Gas, liquid, and solid phases are all present, but not labeled. 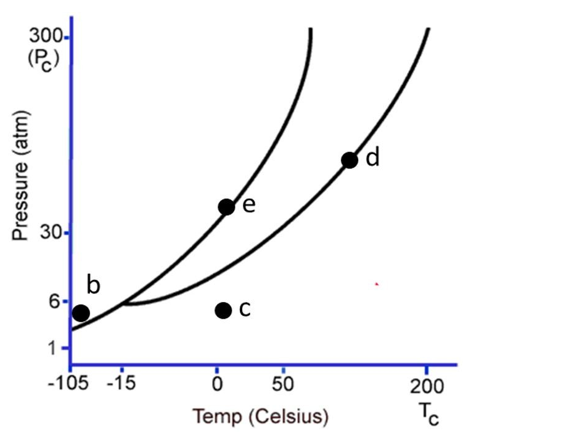 -At the temperature and pressure of point d, which statement below is true?
-At the temperature and pressure of point d, which statement below is true?
(Multiple Choice)
4.7/5  (37)
(37)
Given the phase changes: condensation, freezing, fusion, sublimation, and vaporization. Which of these phase changes is/are endothermic?
(Multiple Choice)
4.8/5  (37)
(37)
Which of the following liquids, at the same temperature, has the lowest vapor pressure?
(Multiple Choice)
4.8/5  (35)
(35)
For small molecules of comparable molecular weight, which one of the following choices lists the intermolecular forces in the order of increasing strength?
(Multiple Choice)
4.8/5  (39)
(39)
Showing 181 - 189 of 189
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)