Exam 12: Intermolecular Attractions and the Properties of Liquids and Solids
Exam 1: A Very Brief History of Chemistry90 Questions
Exam 2: Scientific Measurements224 Questions
Exam 3: Elements, Compounds, and the Periodic Table227 Questions
Exam 4: The Mole and Stoichiometry207 Questions
Exam 5: Molecular View of Reactions in Aqueous Solutions237 Questions
Exam 6: Oxidation-Reduction Reactions175 Questions
Exam 7: Energy and Chemical Change176 Questions
Exam 8: The Quantum Mechanical Atom219 Questions
Exam 9: The Basics of Chemical Bonding167 Questions
Exam 10: Theories of Bonding and Structure196 Questions
Exam 11: Properties of Gases162 Questions
Exam 12: Intermolecular Attractions and the Properties of Liquids and Solids189 Questions
Exam 13: Mixtures at the Molecular Level: Properties of Solutions133 Questions
Exam 14: Chemical Kinetics151 Questions
Exam 15: Chemical Equilibrium109 Questions
Exam 16: Acids and Bases, a Molecular Look104 Questions
Exam 17: Acid-Base Equilibria in Aqueous Solutions184 Questions
Exam 18: Solubility and Simultaneous Equilibria120 Questions
Exam 19: Thermodynamics109 Questions
Exam 20: Electrochemistry143 Questions
Exam 21: Nuclear Reactions and Their Role in Chemistry115 Questions
Exam 22: Metal Complexes113 Questions
Exam 23: Organic Compounds, Polymers, and Biochemicals140 Questions
Select questions type
An unknown solid is hard and brittle with a high melting point. It does not conduct electricity as a solid, but does as a liquid. What kind of crystal best describes this solid?
(Multiple Choice)
4.9/5  (29)
(29)
Dioxane, C4H8O2, has an enthalpy of fusion of 145.8 J g-1 and its melting point temperature is 11.0°C. How much heat is required to transform 142 g of solid dioxane, into liquid dioxane, at 11.0°C?
(Multiple Choice)
4.9/5  (33)
(33)
A unit cell of sodium chloride (face-centered cubic)consists of sodium and chloride ions. How many chloride ions are within this unit cell?
(Multiple Choice)
4.8/5  (41)
(41)
Why would the coffee purchased in a to-go cup with a lid at the fast food takeout shop remain hot much longer than the coffee you pour into your ceramic cup at home?
(Short Answer)
4.7/5  (37)
(37)
The normal boiling point of 2,3,4-trimethypentane, C8H18, is 113.47°C, and its heat of vaporization is 37,600 J mol-1. What is its vapor pressure at 105.5°C?
(Multiple Choice)
4.7/5  (38)
(38)
Arrange the following compounds in order of increasing viscosity: methylene chloride (CH2Cl2); glycerin (C3H5(OH)3); 1,1-ethandiol (C2H4(OH)2); and acetone (C3H6O). Explain your reasoning.
(Essay)
4.8/5  (39)
(39)
A new compound, boganium sulfide, has been discovered. X-ray crystallographic studies reveal that it has a cubic unit cell with a sulfide ion at each of the corner lattice points, a sulfide ion at the geometric center of the unit cell, and a boganium ion in the center of each of the cube faces in the unit cell. Based on this structure, the formula for the compound should be
(Multiple Choice)
4.8/5  (33)
(33)
A solid chemical substance whose triple point pressure is 4.60 atmospheres will normally ________ when it is heated to a sufficiently high temperature in the open laboratory.
(Short Answer)
4.8/5  (27)
(27)
The temperature above which only a solid state and a non-solid exists for a substance is called the ________.
(Short Answer)
4.9/5  (39)
(39)
A technique used for determining the structure of a crystalline substance is
(Multiple Choice)
4.9/5  (27)
(27)
Which of the following would be expected to have the lowest vapor pressure at room temperature?
(Multiple Choice)
4.9/5  (32)
(32)
How many atoms are contained in one unit cell of metallic lanthanum, if it forms a body-centered cubic unit cell?
(Multiple Choice)
4.9/5  (37)
(37)
A unit cell of sodium chloride (face-centered cubic)consists of sodium and chloride ions. How many sodium ions are within this unit cell?
(Multiple Choice)
4.8/5  (40)
(40)
Which set of properties below best describes molecular crystalline substances?
(Multiple Choice)
4.8/5  (39)
(39)
The following questions refer to the phase diagram below. Gas, liquid, and solid phases are all present, but not labeled. 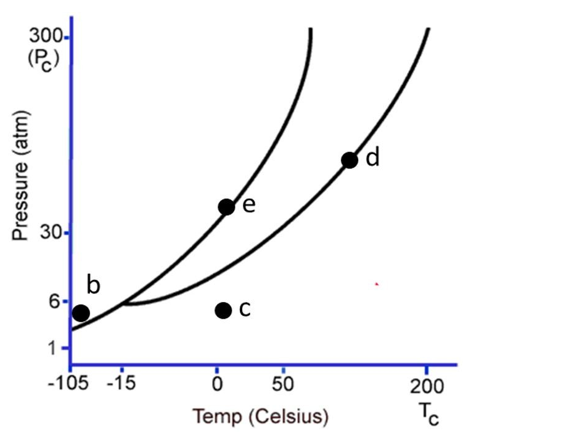 -Starting at the temperature and pressure of b, if the temperature is increased at constant pressure, ultimately
-Starting at the temperature and pressure of b, if the temperature is increased at constant pressure, ultimately
(Multiple Choice)
4.8/5  (31)
(31)
The property that measures or describes the magnitude of resistance to flow in a liquid is called
(Multiple Choice)
4.9/5  (34)
(34)
The following questions refer to the diagram below. 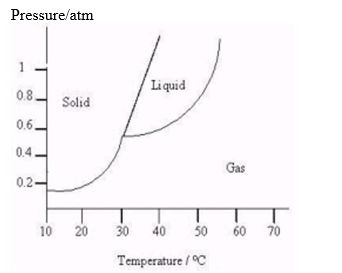 -At what temperatures do the triple point and critical point occur?
-At what temperatures do the triple point and critical point occur?
(Short Answer)
4.8/5  (43)
(43)
Showing 21 - 40 of 189
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)