Exam 8: Effects of Intermolecular Forces
Exam 1: Fundamental Concepts of Chemistry118 Questions
Exam 2: The Behaviour of Gases84 Questions
Exam 3: Energy and Its Conservation65 Questions
Exam 4: Atoms and Light81 Questions
Exam 5: Atomic Energies and Periodicity71 Questions
Exam 6: Fundamentals of Chemical Bonding71 Questions
Exam 7: Theories of Chemical Bonding78 Questions
Exam 8: Effects of Intermolecular Forces71 Questions
Exam 9: Properties of Solutions59 Questions
Exam 10: Organic Chemistrystructure57 Questions
Exam 11: Organic Chemistryreactions40 Questions
Exam 12: Spontaneity of Chemical Processes70 Questions
Exam 13: Kinetics: Mechanisms and Rates of Reactions77 Questions
Exam 14: Principles of Chemical Equilibrium70 Questions
Exam 15: Aqueous Acidbase Equilibria79 Questions
Exam 16: Applications of Aqueous Equilibria66 Questions
Exam 17: Electron Transfer Reactions76 Questions
Exam 18: Macromolecules83 Questions
Exam 19: The Transition Metals38 Questions
Exam 20: The Main Group Elements29 Questions
Exam 21: Nuclear Chemistry and Radiochemistry44 Questions
Select questions type
What is the dominant intermolecular force for propionic acid? 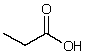
(Multiple Choice)
4.9/5  (31)
(31)
In which of the following pure substances will hydrogen bonding be an important intermolecular force? 1) dichloromethane, CH2Cl2, 2) CH3CH2OH, 3) methylamine, (CH3NH2), 4) trimethylamine, N(CH3)3
(Multiple Choice)
4.8/5  (31)
(31)
Polonium metal crystallizes in a simple cubic structure. If the atomic radius of polonium is 160 pm, what is the volume of a unit cell?
(Multiple Choice)
4.9/5  (41)
(41)
Which of the following experience the strongest covalent bond?
(Multiple Choice)
4.8/5  (41)
(41)
Use the following equation for questions
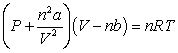 -The value "nb" that is used in the Van der Waals equation accounts for what INCORRECT assumption?
-The value "nb" that is used in the Van der Waals equation accounts for what INCORRECT assumption?
(Multiple Choice)
4.8/5  (36)
(36)
Explain enthalpies of phase changes in terms of intermolecular forces and interpret a pressure-temperature phase diagram of a pure substance.
(Essay)
4.9/5  (36)
(36)
Predict the relative magnitudes of intermolecular forces and their effects on physical properties of substances.
(Essay)
4.9/5  (44)
(44)
Explain the properties of solids in terms of the dominant intermolecular forces present.
(Essay)
4.9/5  (32)
(32)
Some believe that differences between boiling points are truer indicators of relative intermolecular forces than melting points. Why?
(Short Answer)
4.8/5  (34)
(34)
How much energy is required when 23 grams of ethanol at 30˚C are vapourized from a "flaming" desert? ΔH˚vap = 39.3 kJ/mol, Tvap = 351 K, ΔH˚fus = 7.61 kJ/mol, Tfus = 156 K, Cethanol = 112 J/mol˚C
(Short Answer)
4.9/5  (31)
(31)
30 In the simple cubic crystal structure at right, the unit cell is outlined in heavy lines which intersect to form the corners at the center of the spheres. If the corners of the unit cells are at the center of the spheres, how many atoms are in one unit cell? 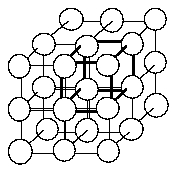
(Multiple Choice)
4.8/5  (36)
(36)
Which of the following experience the strongest intermolecular forces?
(Multiple Choice)
4.8/5  (30)
(30)
Use the phase diagram of oxygen to estimate the temperature at which liquid oxygen will boil under 0.5 atm external pressure and compare that to the normal boiling point. 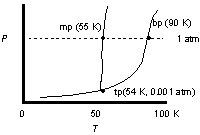
(Short Answer)
4.8/5  (40)
(40)
Which is the most realistic picture for a container of Ar(l)?
(Multiple Choice)
4.9/5  (34)
(34)
The boiling point of HCl (188 K) is lower than that for HI (238 K) because
(Multiple Choice)
4.7/5  (39)
(39)
Where would you expect Ne to appear in the following sequence of boiling points?
He < H2 < N2 < F2 < Ar < O2
(Multiple Choice)
4.8/5  (40)
(40)
List the following three compounds in order of increasing boiling point: 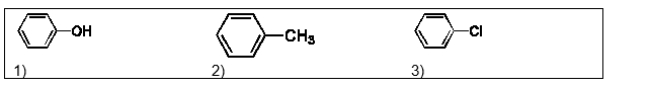
(Multiple Choice)
4.9/5  (35)
(35)
Consider the following three molecules:  The dominant intermolecular force acting in each is, respectively ,
The dominant intermolecular force acting in each is, respectively ,
(Multiple Choice)
4.8/5  (29)
(29)
Of the following pairs, select the pair that undergoes hydrogen bonding and draw a molecular picture of the hydrogen bond interaction.
1. NaCl and acetone, (CH3)2CO
2. CH3OCH3 and CF3Cl
3. NH3 and CCl4
4. NF3 and CF4
5. CH3NH2 and CH3OCH3
(Essay)
4.8/5  (43)
(43)
Showing 41 - 60 of 71
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)