Exam 8: Effects of Intermolecular Forces
Exam 1: Fundamental Concepts of Chemistry118 Questions
Exam 2: The Behaviour of Gases84 Questions
Exam 3: Energy and Its Conservation65 Questions
Exam 4: Atoms and Light81 Questions
Exam 5: Atomic Energies and Periodicity71 Questions
Exam 6: Fundamentals of Chemical Bonding71 Questions
Exam 7: Theories of Chemical Bonding78 Questions
Exam 8: Effects of Intermolecular Forces71 Questions
Exam 9: Properties of Solutions59 Questions
Exam 10: Organic Chemistrystructure57 Questions
Exam 11: Organic Chemistryreactions40 Questions
Exam 12: Spontaneity of Chemical Processes70 Questions
Exam 13: Kinetics: Mechanisms and Rates of Reactions77 Questions
Exam 14: Principles of Chemical Equilibrium70 Questions
Exam 15: Aqueous Acidbase Equilibria79 Questions
Exam 16: Applications of Aqueous Equilibria66 Questions
Exam 17: Electron Transfer Reactions76 Questions
Exam 18: Macromolecules83 Questions
Exam 19: The Transition Metals38 Questions
Exam 20: The Main Group Elements29 Questions
Exam 21: Nuclear Chemistry and Radiochemistry44 Questions
Select questions type
The figure shows the unit cell of a compound containing A (open spheres) and X (shaded spheres). What is the empirical formula of this compound if the shaded spheres form a face-centered cubic arrangement and the open spheres are contained within the unit cell?
(Essay)
4.8/5  (35)
(35)
The face-centered cubic unit cell to the right has been shaded to add clarity; the balls in the corners are black: those in the faces are gray. If the corners of the unit cells are at the center of the corner spheres, how many atoms are in one unit cell? 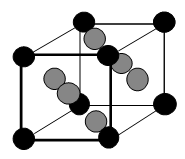
(Multiple Choice)
4.8/5  (42)
(42)
Sketch a phase diagram for hydrazine locating all points given: normal melting point (1.4˚C), normal boiling point (113.5˚C), critical point (380˚C, 145 atm), triple point (2.0˚C, 3.4 mm Hg)
(Essay)
4.7/5  (31)
(31)
Arrange the following in order of increasing vapour pressure at room temperature:
1) H2O,
2) Hg,
3) Br2,
4) CH3CH2OH
(Multiple Choice)
4.8/5  (38)
(38)
Na+ has an ionic radius of 116 pm and Cl- an ionic radius of 167 pm. Estimate the volume of a NaCl unit cell.
(Multiple Choice)
5.0/5  (34)
(34)
The leaves of the lotus plant are extremely hydrophobic. That is, water does not stick to the leaves. In 2004, scientists were able to replicate this phenomenon on a film. Sketch what you think the meniscus of water would look like in a tube made of this material? What would the meniscus look like for oil?
(Essay)
4.9/5  (37)
(37)
Draw molecular pictures that illustrate and explain the different polarizabilities of CH2Cl2 and CHCl3.
(Essay)
4.9/5  (47)
(47)
Below is the structure for zinc sulphide. If the zinc atoms (zinc) are contained within the unit cell and the sulphur atoms (clear) form a face-centered cubic structure, how many sulphur atoms must be contained within the unit cell to balance the charge? 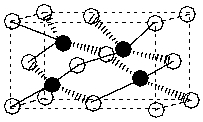
(Multiple Choice)
5.0/5  (32)
(32)
Arrange the following in order of increasing vapour pressure at room temperature.
1) CH3OCH3,
2) CH3CH2OCH2CH3,
3) CH3C(O)CH3,
4) CH3CH2OH
(Multiple Choice)
4.8/5  (31)
(31)
Describe how trees are able to transport water from their roots to the leaves on their branches high in the air.
(Essay)
4.8/5  (44)
(44)
Showing 61 - 71 of 71
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)