Exam 8: Effects of Intermolecular Forces
Exam 1: Fundamental Concepts of Chemistry118 Questions
Exam 2: The Behaviour of Gases84 Questions
Exam 3: Energy and Its Conservation65 Questions
Exam 4: Atoms and Light81 Questions
Exam 5: Atomic Energies and Periodicity71 Questions
Exam 6: Fundamentals of Chemical Bonding71 Questions
Exam 7: Theories of Chemical Bonding78 Questions
Exam 8: Effects of Intermolecular Forces71 Questions
Exam 9: Properties of Solutions59 Questions
Exam 10: Organic Chemistrystructure57 Questions
Exam 11: Organic Chemistryreactions40 Questions
Exam 12: Spontaneity of Chemical Processes70 Questions
Exam 13: Kinetics: Mechanisms and Rates of Reactions77 Questions
Exam 14: Principles of Chemical Equilibrium70 Questions
Exam 15: Aqueous Acidbase Equilibria79 Questions
Exam 16: Applications of Aqueous Equilibria66 Questions
Exam 17: Electron Transfer Reactions76 Questions
Exam 18: Macromolecules83 Questions
Exam 19: The Transition Metals38 Questions
Exam 20: The Main Group Elements29 Questions
Exam 21: Nuclear Chemistry and Radiochemistry44 Questions
Select questions type
You have a solid that it characterized by high melting and boiling points, is NOT conductive, and does NOT dissolve in water. Bonding is most likely
Free
(Multiple Choice)
4.9/5  (40)
(40)
Correct Answer:
C
Acetone CH3COCH3 boils at a significantly higher temperature than 2-methylpropane isobutene
Free
(Multiple Choice)
4.8/5  (39)
(39)
Correct Answer:
E
Understand amorphous and crystalline solids at the molecular level.
Free
(Essay)
4.8/5  (30)
(30)
Correct Answer:
Solid materials may be crystalline or amorphous. The unit cell is the building block of crystalline solids. One way to discuss ionic structures is to identify a crystal lattice for one set of ions and then describe how the other ions pack within the lattice of the first set. Crystalline defects can profoundly alter the properties of a solid.
How much energy is required when 23 grams of ice at -4?C are melted in a 240 ml glass of pop? ?H?vap = 40.7 kJ/mol, ?H?fus = 6.0 kJ/mol, Cice = 37.8 J/mol?C, Cwater = 75.3 J/mol?C
(Short Answer)
4.8/5  (31)
(31)
Use the following equation for questions
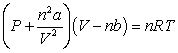 -The relative size of the Van der Waals constant, , correlates well with boiling point; that is, the larger is, the higher the boiling point. The reason for this correlation is
-The relative size of the Van der Waals constant, , correlates well with boiling point; that is, the larger is, the higher the boiling point. The reason for this correlation is
(Multiple Choice)
4.8/5  (33)
(33)
List at least two different physical properties between network and molecular solids.
(Essay)
4.9/5  (34)
(34)
What is the main difference between an amorphous solid and a crystalline solid?
(Essay)
4.8/5  (45)
(45)
Your solid is non-conductive and melts at relatively low temperature. Based on this information, one can conclude that bonding in the solid is most likely
(Multiple Choice)
4.8/5  (40)
(40)
What are the differences in interparticle forces for network and metallic solids?
(Essay)
4.8/5  (43)
(43)
Understand the effects of intermolecular forces on condensation, vapourization, and melting and boiling points.
(Essay)
4.9/5  (34)
(34)
Which is the expected order of increasing boiling point for the following molecules? 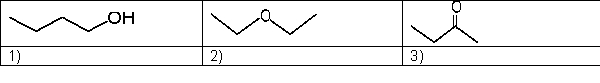
(Multiple Choice)
4.9/5  (40)
(40)
Ruby is a crystalline compound that contains aluminum, oxygen and chromium. The structure of ruby is best described as having
(Multiple Choice)
5.0/5  (44)
(44)
To melt or vapourize a substance, a certain amount of energy needs to be supplied. These are referred to as the heat of fusion and heat of vapourization. Which is typically a lot larger and why?
(Essay)
4.9/5  (33)
(33)
Sketch the phase diagram for benzene identifying the solid, liquid, and gas phases given the following: normal boiling point (80.1˚C), triple point (5.5˚C, 35.8 mm Hg), critical point (288.5˚C and 47.7 atm)
(Essay)
4.7/5  (34)
(34)
Arrange the following in order of decreasing surface tension at room temperature:
1) H2O,
2) Hg,
3) benzene,
4) n-hexane
(Multiple Choice)
4.8/5  (29)
(29)
Explain trends in surface tension, capillary action, viscosity, and vapour pressure in terms of intermolecular forces.
(Essay)
4.9/5  (44)
(44)
The order of increasing melting point for several simple molecular compounds is: H2, F2, O2, N2, Ar, while for these same compounds boiling point increases as H2, N2, F2, Ar, O2. Explain why the trends for boiling point and melting point are different.
(Essay)
4.9/5  (25)
(25)
Which of the following is the expected order of boiling points for H2, He, F2 and Ne?
(Multiple Choice)
4.7/5  (49)
(49)
Showing 1 - 20 of 71
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)