Exam 13: Kinetics: Mechanisms and Rates of Reactions
Exam 1: Fundamental Concepts of Chemistry118 Questions
Exam 2: The Behaviour of Gases84 Questions
Exam 3: Energy and Its Conservation65 Questions
Exam 4: Atoms and Light81 Questions
Exam 5: Atomic Energies and Periodicity71 Questions
Exam 6: Fundamentals of Chemical Bonding71 Questions
Exam 7: Theories of Chemical Bonding78 Questions
Exam 8: Effects of Intermolecular Forces71 Questions
Exam 9: Properties of Solutions59 Questions
Exam 10: Organic Chemistrystructure57 Questions
Exam 11: Organic Chemistryreactions40 Questions
Exam 12: Spontaneity of Chemical Processes70 Questions
Exam 13: Kinetics: Mechanisms and Rates of Reactions77 Questions
Exam 14: Principles of Chemical Equilibrium70 Questions
Exam 15: Aqueous Acidbase Equilibria79 Questions
Exam 16: Applications of Aqueous Equilibria66 Questions
Exam 17: Electron Transfer Reactions76 Questions
Exam 18: Macromolecules83 Questions
Exam 19: The Transition Metals38 Questions
Exam 20: The Main Group Elements29 Questions
Exam 21: Nuclear Chemistry and Radiochemistry44 Questions
Select questions type
Explain the concepts of a mechanism and a rate-determining step in a chemical reaction.
Free
(Essay)
4.8/5  (36)
(36)
Correct Answer:
A reaction mechanism is the exact molecular pathway that starting materials follow on their way to becoming products. The overall reaction is the sum of the elementary steps in the mechanism. The rate-determining step is the slowest step in the reaction mechanism. The overall reaction cannot go faster than the rate-determining step.
The reaction of ozone with oxygen atoms to produce molecular oxygen has a rate constant of 4.8x106 1/M s at 25oC. A 20 degree increase in temperature results in a rate constant of 7.4x106 1/M s. What is the rate constant at 100oC?
Free
(Short Answer)
4.8/5  (44)
(44)
Correct Answer:
1.9x107 1/M s
Determine rate laws from concentration versus time data.
Free
(Essay)
4.7/5  (34)
(34)
Correct Answer:
Rate laws must be determined from experimental data. The half-life of a first-order reaction is a constant. The half-life of a second-order reaction depends on the initial concentration.
A proposed mechanism for the following reaction, A2 + B2 2AB, isA2  2AA + B2 AB + B slowB +A2 AB + ADetermine the rate law.
2AA + B2 AB + B slowB +A2 AB + ADetermine the rate law.
(Short Answer)
4.8/5  (41)
(41)
Nitrous oxide, N2O, decomposes on metal surfaces readily at high temperatures following first-order kinetics for the equation:
2 N2O (g) 2 N2 (g) + O2 (g)
The following data are obtained for a reaction at 850°C: 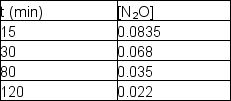 What was the initial concentration of N2O (t = 0)?
What was the initial concentration of N2O (t = 0)?
(Multiple Choice)
4.7/5  (27)
(27)
The following reaction takes place at 80.1°C:
Ru(NH3)5Cl2+ (aq) + H2O (l) Ru(NH3)5(H2O)3+ (aq) + Cl- (aq)
The following time and concentration data are collected: 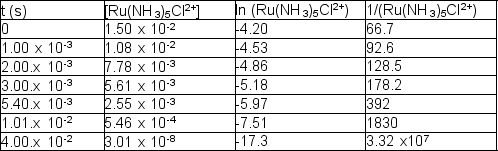 Which of the following is the correct value of the rate constant?
Which of the following is the correct value of the rate constant?
(Multiple Choice)
4.8/5  (38)
(38)
Trioxane undergoes decomposition to formaldehyde at elevated temperatures.C3H6O3 (g) 3 CH2O (g)The following data was collected for the gas phase reaction at 519ºK: 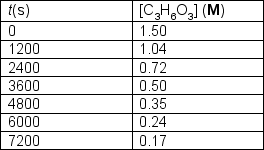 Determine the order of the decomposition in trioxane and the rate constant at 519ºK.
Determine the order of the decomposition in trioxane and the rate constant at 519ºK.
(Short Answer)
4.7/5  (32)
(32)
You are running late for a basketball game on your campus and you are thinking about what will be the rate determining step for attending the basketball game? Which of the following will be your rate determining step?
(Multiple Choice)
4.8/5  (47)
(47)
The following are initial rate data for 2 NO + 2 H2 N2 + 2 H2O ![The following are initial rate data for 2 NO + 2 H<sub>2</sub> \rightarrow N<sub>2</sub> + 2 H<sub>2</sub>O The rate law is determined to be: rate = k[NO]<sup>2</sup>[H<sub>2</sub>]. With this information determine k using the data from experiment 2.](https://storage.examlex.com/TB9687/11ee726d_d443_5bd4_827e_b997e40295b0_TB9687_00.jpg) The rate law is determined to be: rate = k[NO]2[H2]. With this information determine k using the data from experiment 2.
The rate law is determined to be: rate = k[NO]2[H2]. With this information determine k using the data from experiment 2.
(Short Answer)
4.9/5  (40)
(40)
Butadiene reacts to form its dimmer according to the following reaction: ![Butadiene reacts to form its dimmer according to the following reaction: Concentration versus time data were collected for this reaction and a plot of 1/[C<sub>4</sub>H<sub>6</sub>] resulted in a straight line with slope 6.14 x10<sup>-2</sup> M s<sup>-1</sup>. The integrated for of the rate law is](https://storage.examlex.com/TB9687/11ee726d_d441_60e8_827e_69ce9e45369e_TB9687_00.jpg) Concentration versus time data were collected for this reaction and a plot of 1/[C4H6] resulted in a straight line with slope 6.14 x10-2 M s-1. The integrated for of the rate law is
Concentration versus time data were collected for this reaction and a plot of 1/[C4H6] resulted in a straight line with slope 6.14 x10-2 M s-1. The integrated for of the rate law is
(Multiple Choice)
4.8/5  (37)
(37)
The industrial production of 2-propanol involves the reaction of propene with sulphuric acid and then water. Write the second step of the mechanism if the following is the first step: 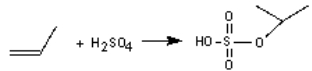
(Essay)
4.8/5  (45)
(45)
The reaction of ozone with oxygen atoms to give oxygen has an activation energy of 17.1 kJ/mole with a rate constant at 298oK, k = 4.8 x 106 1/M•s. Calculate the rate constant for this reaction at 315oK.
(Short Answer)
4.7/5  (34)
(34)
Consider the aqueous phase reaction between the dichromate anion and iron (II) cations:14 H3O+(aq) + Cr2O72- + 6Fe2+(aq) 2Cr3+(aq) + 21H2OWhat is the reaction rate expressed in terms of changing H3O+ concentration?
(Multiple Choice)
4.8/5  (39)
(39)
Determine the rate law, given the mechanism and knowledge of the relative rates of steps of a reaction.
(Essay)
4.8/5  (33)
(33)
Assume that the following first-order reaction has a rate constant k = 0.0137/min:
SO2Cl2 SO2 + Cl2
Given the initial [SO2Cl2] = 0.42 M, how many minutes will it take for [SO2Cl2] = 0.19 M?
(Short Answer)
4.9/5  (35)
(35)
Consider the aqueous phase reaction between hydrogen gas and liquid bromine:H2(g) + Br2(g) 2HBr(g)Which of the following expressions accurately express the rate of the above reaction?
I. Reaction rate = 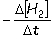 II. Reaction rate =
II. Reaction rate = 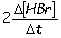 III. Reaction rate =
III. Reaction rate = 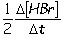 IV. Reaction rate =
IV. Reaction rate = 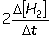
(Multiple Choice)
4.8/5  (43)
(43)
The rate constant of the reaction, O (g) + N2 (g) NO (g) + N (g), is 9.7 x 10101/M•s at 800oK and has an activation energy of 315 kJ/mole. What is the value of the rate constant at 700oK?
(Short Answer)
4.8/5  (30)
(30)
The following rate constants were obtained at the stated temperatures for the first-order reaction:A B  Find the activation energy (in kJ/mole) for this reaction.
Find the activation energy (in kJ/mole) for this reaction.
(Short Answer)
4.9/5  (41)
(41)
It has been suggested that the decomposition of NO2 occurs via the following mechanism:
NO2 NO + O (Rxn I)
O + NO2 2NO + O2 (Rxn II)
Predict the rate determining step.
(Multiple Choice)
4.9/5  (41)
(41)
Showing 1 - 20 of 77
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)