Exam 17: Electron Transfer Reactions
Exam 1: Fundamental Concepts of Chemistry118 Questions
Exam 2: The Behaviour of Gases84 Questions
Exam 3: Energy and Its Conservation65 Questions
Exam 4: Atoms and Light81 Questions
Exam 5: Atomic Energies and Periodicity71 Questions
Exam 6: Fundamentals of Chemical Bonding71 Questions
Exam 7: Theories of Chemical Bonding78 Questions
Exam 8: Effects of Intermolecular Forces71 Questions
Exam 9: Properties of Solutions59 Questions
Exam 10: Organic Chemistrystructure57 Questions
Exam 11: Organic Chemistryreactions40 Questions
Exam 12: Spontaneity of Chemical Processes70 Questions
Exam 13: Kinetics: Mechanisms and Rates of Reactions77 Questions
Exam 14: Principles of Chemical Equilibrium70 Questions
Exam 15: Aqueous Acidbase Equilibria79 Questions
Exam 16: Applications of Aqueous Equilibria66 Questions
Exam 17: Electron Transfer Reactions76 Questions
Exam 18: Macromolecules83 Questions
Exam 19: The Transition Metals38 Questions
Exam 20: The Main Group Elements29 Questions
Exam 21: Nuclear Chemistry and Radiochemistry44 Questions
Select questions type
Nitrogen has many possible oxidation numbers; put the following nitrogen compounds in order of increasing oxidation number: NO2, HNO3, NO2-, NO.
Free
(Multiple Choice)
4.8/5  (38)
(38)
Correct Answer:
D
Which of the species listed is the strongest oxidizing agent?  (If needed, refer to Table 17-1 in the text)
(If needed, refer to Table 17-1 in the text)
Free
(Short Answer)
4.9/5  (41)
(41)
Correct Answer:
Pt+2
Consider an electrochemical cell consisting of an Fe(s) electrode, Fe(NO3)2 electrolyte connected through a salt bridge to a Ag wire coated in AgCl(s) immersed in an aqueous KCl solution. Is the standard cell and balanced galvanic cell reaction:(If needed, refer to Table 17-1 in the text )
Free
(Multiple Choice)
4.8/5  (45)
(45)
Correct Answer:
B
Balance the following half reaction under acidic conditions:OCl- Cl-
(Short Answer)
4.8/5  (35)
(35)
An electrochemical cell is constructed that contains Cr3+(aq) and Cr metal as the electrode in one compartment and Cu2+(aq) and copper metal in the other compartment. Calculate the expected standard potential upon appropriately connecting the cell and describe the direction of electron and cation flow.(If needed, refer to Table 17-1 in the text)
(Essay)
5.0/5  (42)
(42)
For the reaction given below, which half reaction occurs at the cathode?Pb(s) + PbO2(s) + 2HSO4-(aq) + 2H3O+(aq) 2PbSO4(aq) +4H2O(l)
(Multiple Choice)
4.7/5  (44)
(44)
Which of the species listed is the strongest reducing agent? 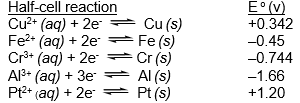 (If needed, refer to Table 17-1 in the text )
(If needed, refer to Table 17-1 in the text )
(Short Answer)
4.8/5  (43)
(43)
The following reaction occurs in a galvanic cell:
NiO2 + Cd + H2O Cd(OH)2 + Ni(OH)2 + 2 OH-Which redox process in this battery occurs at a passive electrode?
(Multiple Choice)
4.8/5  (41)
(41)
Draw a figure illustrating how a cell would be arranged for the redox reaction of copper with silver ion but using indirect electron transfer and a salt bridge with KNO3 solution. Indicate the direction of electron flow in the wire and the movement of ions in the salt bridge.
(Essay)
5.0/5  (36)
(36)
Balance the following half reaction under basic conditions:
MnO4- MnO2(s)
(Short Answer)
4.9/5  (35)
(35)
Consider an automobile which is powered by a perfectly efficient fuel cell that consumes hydrogen and oxygen in the following redox reaction:
2H2(g) + O2(g) 2 H2O (l)If the electric system requires a current of 500 amperes, how many g of H2 are consumed per hour?(If needed, refer to Table 17-1 in the text )
(Short Answer)
4.9/5  (34)
(34)
Consider an electrochemical cell of the type shown in the figure where the redox half-reaction in both compartments has the identical standard potentials: 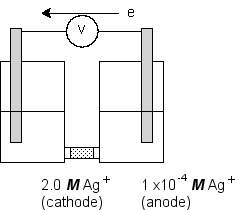 Use the Nernst equation to calculate the potential developed by this cell.(If needed, refer to Table 17-1 in the text)
Use the Nernst equation to calculate the potential developed by this cell.(If needed, refer to Table 17-1 in the text)
(Short Answer)
4.8/5  (35)
(35)
For a brine electrolysis cell (see redox reaction below) operating at 60,000 amps, how many kg of NaOH and Cl2 would be produced in 24.0 hours?
2 NaCl (aq) + 2 H2O 2 NaOH (aq) + Cl2(g) + H2 (g)
(Short Answer)
4.8/5  (34)
(34)
Platinum metal is quite resistant to oxidation as may be deduced by its reduction potential:Pt2+ + 2e Pt E° 1.2 VExamine a table of reduction potentials (Table 17-1 in the text) and determine two elements capable of oxidizing platinum under standard conditions.
(Multiple Choice)
4.8/5  (41)
(41)
Showing 1 - 20 of 76
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)