Exam 2: The Behaviour of Gases
Exam 1: Fundamental Concepts of Chemistry118 Questions
Exam 2: The Behaviour of Gases84 Questions
Exam 3: Energy and Its Conservation65 Questions
Exam 4: Atoms and Light81 Questions
Exam 5: Atomic Energies and Periodicity71 Questions
Exam 6: Fundamentals of Chemical Bonding71 Questions
Exam 7: Theories of Chemical Bonding78 Questions
Exam 8: Effects of Intermolecular Forces71 Questions
Exam 9: Properties of Solutions59 Questions
Exam 10: Organic Chemistrystructure57 Questions
Exam 11: Organic Chemistryreactions40 Questions
Exam 12: Spontaneity of Chemical Processes70 Questions
Exam 13: Kinetics: Mechanisms and Rates of Reactions77 Questions
Exam 14: Principles of Chemical Equilibrium70 Questions
Exam 15: Aqueous Acidbase Equilibria79 Questions
Exam 16: Applications of Aqueous Equilibria66 Questions
Exam 17: Electron Transfer Reactions76 Questions
Exam 18: Macromolecules83 Questions
Exam 19: The Transition Metals38 Questions
Exam 20: The Main Group Elements29 Questions
Exam 21: Nuclear Chemistry and Radiochemistry44 Questions
Select questions type
The ideal gas equation is expected to be valid at
Free
(Multiple Choice)
4.8/5  (37)
(37)
Correct Answer:
B
In this chapter, some important applications of high vacuum are discussed. Pressures of 1.0 x 10-9 atm can be routinely achieved in the laboratory. CO is a common contaminant at these low levels. How many molecules of CO would be present if it was the only contaminant in a 1.0 L container at a pressure of 1.0 x 10-9 atm and 298 K?
Free
(Short Answer)
4.9/5  (38)
(38)
Correct Answer:
2.5 x 1013
Consider the van der Waals "b" coefficient for He, O2 and CO2 increases because
Free
(Multiple Choice)
4.9/5  (49)
(49)
Correct Answer:
D
A hair spray bottle will explode if under 9 atm of internal pressure. If this bottle is originally under 5 atm of pressure at 25˚C and is placed to close to a campfire and raises to 48˚C, what will be the pressure inside the bottle now?
(Short Answer)
4.8/5  (40)
(40)
If atmospheric pressure atop Mount Everest is about 250 mm Hg, what is the partial pressure of oxygen?
(Short Answer)
4.8/5  (40)
(40)
A gas mixture containing oxygen, nitrogen, and helium; if the partial pressure and mole fraction of oxygen is 425 Torr and 0.46, respectively, and the partial pressure of helium is 74 Torr, what is the partial pressure (in bar) of nitrogen in the mixture?
(Short Answer)
5.0/5  (26)
(26)
Consider a sample of gas in a tank. Under which of the following conditions would you expect the least ideal behaviour?
(Multiple Choice)
4.7/5  (36)
(36)
If the relative humidity at 28°C is 58.3 %, what is the dew point? 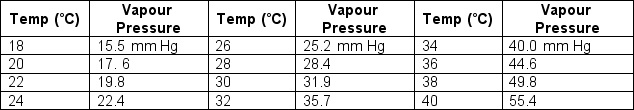
(Short Answer)
4.9/5  (47)
(47)
The level of mercury on the sample side of a manometer is 56 mm below that of the atmospheric side. If the atmospheric pressure is 1.05 bar, what is the pressure of the gas sample (in bar)?
(Short Answer)
4.8/5  (34)
(34)
A sample of gas is cooled to its vapourization temperature. What is true before the sample reaches equilibrium between the liquid and gaseous phases?
(Multiple Choice)
4.9/5  (40)
(40)
Consider the van der Waals "a" coefficient for He, O2 and C6H6 increases because
(Multiple Choice)
4.9/5  (28)
(28)
Which will occupy a larger volume, 10 moles of H2 (g) or 10 moles of propane, C3H8 (g)?
(Multiple Choice)
4.9/5  (46)
(46)
If the compressibility factor, pV/nRT is 1 at pressure p, then
(Multiple Choice)
4.8/5  (41)
(41)
Methylamine, whose line structure is shown below, is one of the substances for the "fishy" odour of not-so-fresh fish.  What is the average velocity and average kinetic energy (kJ/mole) of gaseous methylamine molecules at 25°C?
What is the average velocity and average kinetic energy (kJ/mole) of gaseous methylamine molecules at 25°C?
(Short Answer)
4.7/5  (32)
(32)
If 760 Torr is equivalent to 1 atm and to 1.01325 bar, then a pressure of 1 bar is equivalent to
(Multiple Choice)
4.9/5  (40)
(40)
A glass bulb with a volume of 250.0 mL contains a small amount of solid potassium chlorate. It is connected to a mercury manometer. Upon heating the bulb, the following (unbalanced) reaction occurs:
KClO3 KCl + O2
After the apparatus cools back to room temperature, 23°C, the difference in the mercury levels in the manometer is now 14.2 cm. Which of the following statements are true?
1) The mercury on the side of the tube connected to the manometer is higher than before.
2) About 1.9 x 10-3 moles KClO3 decomposed.
3) About 0.16 g KClO3 decomposed.
4) The mercury on the side of the tube connected to the manometer is lower than before.
5) About 1.3 x 10-3 moles O2 were evolved.
(Multiple Choice)
4.9/5  (31)
(31)
The noxious gas H2S can be used to remove the acid rain component SO2 from factory emissions by the Claus Process. What volume of H2S in litres at STP will be needed to remove 2.00 kg of SO2?
2 H2S(g) + SO2(g) 3 S(s) + 2 H2O(l)
(Multiple Choice)
4.9/5  (41)
(41)
Do calculations involving water vapour pressure and relative humidity and describe some of the basic chemistry of the troposphere.
(Essay)
4.9/5  (33)
(33)
Showing 1 - 20 of 84
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)