Exam 18: Two Classes of Chemical Reactions
Exam 1: Patterns of Motion and Equilibrium126 Questions
Exam 2: Newtons Laws of Motion127 Questions
Exam 3: Momentum and Energy181 Questions
Exam 4: Gravity, Projectiles, and Satellites147 Questions
Exam 5: Fluid Mechanics148 Questions
Exam 6: Thermal Energy and Thermodynamics113 Questions
Exam 7: Heat Transfer and Change of Phase158 Questions
Exam 8: Static and Current Electricity178 Questions
Exam 9: Magnetism and Electromagnetic Induction130 Questions
Exam 10: Waves and Sound146 Questions
Exam 11: Light163 Questions
Exam 12: Atoms and the Periodic Table137 Questions
Exam 13: The Atomic Nucleus and Radioactivity127 Questions
Exam 14: Elements of Chemistry69 Questions
Exam 15: How Atoms Bond and Molecules Attract150 Questions
Exam 16: Mixtures141 Questions
Exam 17: How Chemicals React120 Questions
Exam 18: Two Classes of Chemical Reactions177 Questions
Exam 19: Organic Compounds96 Questions
Exam 20: Rocks and Minerals169 Questions
Exam 21: Plate Tectonics and Earths Interior181 Questions
Exam 22: Shaping Earths Surface180 Questions
Exam 23: Geologic Time - Reading the Rock Record167 Questions
Exam 24: The Oceans, Atmosphere, and Climatic Effects188 Questions
Exam 25: Driving Forces of Weather166 Questions
Exam 26: The Solar System108 Questions
Exam 27: Stars and Galaxies107 Questions
Exam 28: The Structure of Space and Time73 Questions
Exam 29: Prologue: The Nature of Science22 Questions
Select questions type
A major source of chlorine gas, ,is from the electrolysis of brine,which is concentrated salt water, What is the sign of the electrode where the chlorine gas is formed? Is it negative or positive?
(Multiple Choice)
4.7/5  (30)
(30)
How many electrons are gained or lost in the following half-reaction? 2 Na → 2 Na+
(Multiple Choice)
4.8/5  (35)
(35)
When the hydronium ion concentration equals 1 mole per liter,what is the pH of the solution? Is the solution acidic or basic?
(Multiple Choice)
4.9/5  (36)
(36)
Which of the following materials is most likely to act as an oxidizing agent?
(Multiple Choice)
4.9/5  (37)
(37)
Qualitatively,what happens to the hydroxide ion concentration if you decrease the hydronium ion concentration?
(Multiple Choice)
4.7/5  (42)
(42)
If the pH of a solution was 7 and you were to increase the hydronium ion concentration 1000x,what would the pH be?
(Multiple Choice)
4.7/5  (31)
(31)
Arrange the following images of an aqueous base solution in order of increasing base strength: 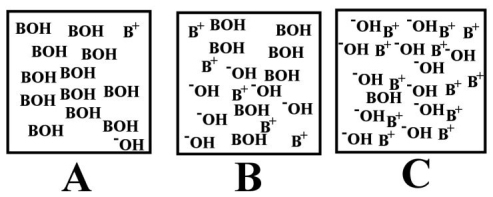
(Multiple Choice)
4.8/5  (33)
(33)
When a hydronium ion concentration equals 1 × moles per liter,what is the pH of the solution? Is the solution acidic or basic?
(Multiple Choice)
4.8/5  (37)
(37)
Why might a small piece of chalk made of calcium carbonate be useful for alleviating acid indigestion?
(Multiple Choice)
4.8/5  (42)
(42)
What might the relationship be between an element's electronegativity and its ability to behave as an oxidizing agent?
(Multiple Choice)
4.8/5  (34)
(34)
Why is carbon dioxide able to be stored more effectively in ocean water vs.fresh water?
(Multiple Choice)
4.9/5  (40)
(40)
Which of the half-reactions below would be complementary,balance the following half-reaction,and be chemically reasonable? Br2 → 2 Br-
(Multiple Choice)
4.8/5  (31)
(31)
A major source of chlorine gas, ,is from the electrolysis of brine,which is concentrated salt water, Which of the following is the balanced chemical reaction for this electrolysis reaction?
(Multiple Choice)
4.9/5  (29)
(29)
Showing 41 - 60 of 177
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)