Exam 18: Two Classes of Chemical Reactions
Exam 1: Patterns of Motion and Equilibrium126 Questions
Exam 2: Newtons Laws of Motion127 Questions
Exam 3: Momentum and Energy181 Questions
Exam 4: Gravity, Projectiles, and Satellites147 Questions
Exam 5: Fluid Mechanics148 Questions
Exam 6: Thermal Energy and Thermodynamics113 Questions
Exam 7: Heat Transfer and Change of Phase158 Questions
Exam 8: Static and Current Electricity178 Questions
Exam 9: Magnetism and Electromagnetic Induction130 Questions
Exam 10: Waves and Sound146 Questions
Exam 11: Light163 Questions
Exam 12: Atoms and the Periodic Table137 Questions
Exam 13: The Atomic Nucleus and Radioactivity127 Questions
Exam 14: Elements of Chemistry69 Questions
Exam 15: How Atoms Bond and Molecules Attract150 Questions
Exam 16: Mixtures141 Questions
Exam 17: How Chemicals React120 Questions
Exam 18: Two Classes of Chemical Reactions177 Questions
Exam 19: Organic Compounds96 Questions
Exam 20: Rocks and Minerals169 Questions
Exam 21: Plate Tectonics and Earths Interior181 Questions
Exam 22: Shaping Earths Surface180 Questions
Exam 23: Geologic Time - Reading the Rock Record167 Questions
Exam 24: The Oceans, Atmosphere, and Climatic Effects188 Questions
Exam 25: Driving Forces of Weather166 Questions
Exam 26: The Solar System108 Questions
Exam 27: Stars and Galaxies107 Questions
Exam 28: The Structure of Space and Time73 Questions
Exam 29: Prologue: The Nature of Science22 Questions
Select questions type
Which of the half-reactions below would be complementary and balance the following half-reaction? Zn+2 → Zn
(Multiple Choice)
4.9/5  (35)
(35)
How would connecting iron with a wire to a piece of metal like copper,which undergoes reduction very easily,affect the rate at which the iron corrodes?
(Multiple Choice)
4.9/5  (31)
(31)
How might you tell whether or not your toothpaste contained either calcium carbonate,CaC ,or baking soda,NaHC ,without looking at the ingredients label?
(Multiple Choice)
4.9/5  (35)
(35)
Which of the following materials is most likely to undergo oxidation?
(Multiple Choice)
4.9/5  (43)
(43)
For the following electrolysis reaction: 2 AlOF3-2 + C → 2 Al + CO2 + F-
Where would the aluminum form?
(Multiple Choice)
4.9/5  (38)
(38)
How many electrons are gained or lost in the following half-reaction? Cl2 → 2 Cl-
(Multiple Choice)
4.8/5  (30)
(30)
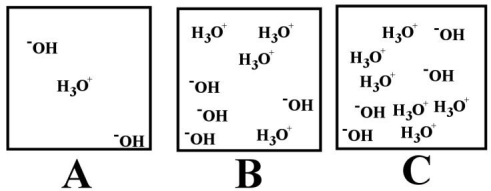 -Which of the above illustrations shows a basic aqueous solution?
-Which of the above illustrations shows a basic aqueous solution?
(Multiple Choice)
4.8/5  (33)
(33)
What is the hydroxide ion concentration in an aqueous solution when the hydronium ion concentration equals 1 × moles per liter?
(Multiple Choice)
4.7/5  (30)
(30)
In a battery,if the following oxidation-reduction reactions takes place normally: Mn2O3 + ZnO → 2 MnO2 + Zn
What reaction would be taking place if you were charging the battery?
(Multiple Choice)
4.9/5  (34)
(34)
What would the concentration of H3O+ be if the concentration of OH- was 1 × 10-11M? [H3O+] × [OH-] = Kw = 1 × 10-14
(Multiple Choice)
4.9/5  (39)
(39)
Cutting back on the pollutants that cause acid rain is one solution to the problem of acidified lakes.Suggest another.
(Multiple Choice)
4.7/5  (29)
(29)
Which of the following reactions illustrates an amphoteric compound?
(Multiple Choice)
4.7/5  (40)
(40)
What best describes what happens when an acid such as HCl is mixed with water?
(Multiple Choice)
4.8/5  (35)
(35)
What do the brackets in the following equation represent? [H3O+] × [OH-] = Kw
(Multiple Choice)
4.7/5  (37)
(37)
 -Which of the above illustrations shows a neutral aqueous solution?
-Which of the above illustrations shows a neutral aqueous solution?
(Multiple Choice)
4.8/5  (29)
(29)
When a hydronium ion concentration equals 1 × moles per liter,what is the pH of the solution? Is the solution acidic or basic?
(Multiple Choice)
4.7/5  (36)
(36)
Which of the following species is undergoing reduction? 2 CuBr → 2Cu + Br2
(Multiple Choice)
4.7/5  (37)
(37)
Showing 61 - 80 of 177
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)