Exam 18: Two Classes of Chemical Reactions
Exam 1: Patterns of Motion and Equilibrium126 Questions
Exam 2: Newtons Laws of Motion127 Questions
Exam 3: Momentum and Energy181 Questions
Exam 4: Gravity, Projectiles, and Satellites147 Questions
Exam 5: Fluid Mechanics148 Questions
Exam 6: Thermal Energy and Thermodynamics113 Questions
Exam 7: Heat Transfer and Change of Phase158 Questions
Exam 8: Static and Current Electricity178 Questions
Exam 9: Magnetism and Electromagnetic Induction130 Questions
Exam 10: Waves and Sound146 Questions
Exam 11: Light163 Questions
Exam 12: Atoms and the Periodic Table137 Questions
Exam 13: The Atomic Nucleus and Radioactivity127 Questions
Exam 14: Elements of Chemistry69 Questions
Exam 15: How Atoms Bond and Molecules Attract150 Questions
Exam 16: Mixtures141 Questions
Exam 17: How Chemicals React120 Questions
Exam 18: Two Classes of Chemical Reactions177 Questions
Exam 19: Organic Compounds96 Questions
Exam 20: Rocks and Minerals169 Questions
Exam 21: Plate Tectonics and Earths Interior181 Questions
Exam 22: Shaping Earths Surface180 Questions
Exam 23: Geologic Time - Reading the Rock Record167 Questions
Exam 24: The Oceans, Atmosphere, and Climatic Effects188 Questions
Exam 25: Driving Forces of Weather166 Questions
Exam 26: The Solar System108 Questions
Exam 27: Stars and Galaxies107 Questions
Exam 28: The Structure of Space and Time73 Questions
Exam 29: Prologue: The Nature of Science22 Questions
Select questions type
Copper atoms have a greater tendency to be reduced than iron atoms do.Was this good news or bad news for the Statue of Liberty,whose copper exterior was originally held together by steel rivets?
(Multiple Choice)
4.9/5  (26)
(26)
For the following acid-base reaction,identify which salt is formed. HCl + NaOH ⇌ ???? + H2O
(Multiple Choice)
4.7/5  (37)
(37)
Aluminum metal undergoes the same basic corrosion process that iron does yet it does not decompose as rapidly.Why?
(Multiple Choice)
4.9/5  (37)
(37)
Water is formed from the reaction of an acid and a base.Why is it not classified as a salt?
(Multiple Choice)
4.8/5  (34)
(34)
Which of the following species is the reducing agent? 2 CuBr → 2Cu + Br2
(Multiple Choice)
4.7/5  (36)
(36)
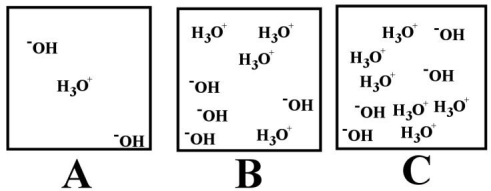 -Which of the above illustrations shows an acidic aqueous solution?
-Which of the above illustrations shows an acidic aqueous solution?
(Multiple Choice)
4.8/5  (34)
(34)
What element is behaves as the oxidizing agent in the following equation and what element behaves as the reducing agent? + 2 Ag ? Sn + 2 Ag?
(Multiple Choice)
4.8/5  (31)
(31)
For each of the following unbalanced equations,which is depicted: oxidation or reduction? a)Cr ? b) Sn ?
(Multiple Choice)
4.9/5  (27)
(27)
Why might disposing of a lead-acid battery,nickel-cadmium battery or mercury battery in a landfill be a bad thing?
(Multiple Choice)
4.7/5  (35)
(35)
What happens to the corrosive properties of an acid and a base after they neutralize each other? Why?
(Multiple Choice)
4.8/5  (29)
(29)
Which of the following statements about strong and weak bases is not true?
(Multiple Choice)
4.9/5  (35)
(35)
Why does a battery that has thick zinc walls last longer than one that has thin zinc walls?
(Multiple Choice)
4.9/5  (33)
(33)
Which of the following statements about strong or weak bases is true?
(Multiple Choice)
4.8/5  (38)
(38)
Pour vinegar onto beach sand from the Caribbean and the result is a lot of froth and bubbles.Pour vinegar onto beach sand from California,however,and nothing happens.Why?
(Multiple Choice)
4.9/5  (32)
(32)
When lightning strikes,nitrogen molecules, ,and oxygen molecules, ,in the air react to form nitrates, ?,which come down in the rain to help fertilize the soil.Is this this an example of oxidation or reduction?
(Multiple Choice)
4.7/5  (32)
(32)
Which of the following statements describes an acidic solution?
(Multiple Choice)
4.8/5  (36)
(36)
What would be the best explanation for the fact that most natural water has a pH of about 5.6?
(Multiple Choice)
4.8/5  (38)
(38)
The general chemical equation for photosynthesis is shown below.Through this reaction are the oxygens of the water molecules,H2O,oxidized or reduced? 6 + 6 O ? + 6
(Multiple Choice)
4.9/5  (26)
(26)
According to the following reaction,which molecule is acting as a base? OH- + NH4+ → H2O + NH3
(Multiple Choice)
4.8/5  (39)
(39)
The following set of redox reactions takes place when iron is dipped in a solution of copper ions. Fe → Fe+2 + 2e-
Cu+2 + 2e- → Cu
Which of the following describes what is happening on the surface of the iron?
(Multiple Choice)
4.9/5  (40)
(40)
Showing 141 - 160 of 177
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)