Exam 18: Two Classes of Chemical Reactions
Exam 1: Patterns of Motion and Equilibrium126 Questions
Exam 2: Newtons Laws of Motion127 Questions
Exam 3: Momentum and Energy181 Questions
Exam 4: Gravity, Projectiles, and Satellites147 Questions
Exam 5: Fluid Mechanics148 Questions
Exam 6: Thermal Energy and Thermodynamics113 Questions
Exam 7: Heat Transfer and Change of Phase158 Questions
Exam 8: Static and Current Electricity178 Questions
Exam 9: Magnetism and Electromagnetic Induction130 Questions
Exam 10: Waves and Sound146 Questions
Exam 11: Light163 Questions
Exam 12: Atoms and the Periodic Table137 Questions
Exam 13: The Atomic Nucleus and Radioactivity127 Questions
Exam 14: Elements of Chemistry69 Questions
Exam 15: How Atoms Bond and Molecules Attract150 Questions
Exam 16: Mixtures141 Questions
Exam 17: How Chemicals React120 Questions
Exam 18: Two Classes of Chemical Reactions177 Questions
Exam 19: Organic Compounds96 Questions
Exam 20: Rocks and Minerals169 Questions
Exam 21: Plate Tectonics and Earths Interior181 Questions
Exam 22: Shaping Earths Surface180 Questions
Exam 23: Geologic Time - Reading the Rock Record167 Questions
Exam 24: The Oceans, Atmosphere, and Climatic Effects188 Questions
Exam 25: Driving Forces of Weather166 Questions
Exam 26: The Solar System108 Questions
Exam 27: Stars and Galaxies107 Questions
Exam 28: The Structure of Space and Time73 Questions
Exam 29: Prologue: The Nature of Science22 Questions
Select questions type
According to the following reaction,which molecule is acting as an acid? H2O + H2SO4 → H3O+ + HSO4-
(Multiple Choice)
4.9/5  (36)
(36)
Water is 88.88 percent oxygen by mass.Oxygen is exactly what a fire needs to grow brighter and stronger.So why doesn't a fire grow brighter and stronger when water is added to it?
(Multiple Choice)
4.9/5  (44)
(44)
The following set of redox reactions takes place when iron is dipped into a solution of copper ions. Fe → Fe+2 + 2e-
Cu+2 + 2e- → Cu
Which of the following describes what is happening with the electrons in the solution?
(Multiple Choice)
4.8/5  (40)
(40)
Which of the following materials is most likely to undergo reduction?
(Multiple Choice)
4.8/5  (38)
(38)
Sodium hydroxide,NaOH,is a strong base,which means that it readily accepts hydrogen ions.What products are formed when sodium hydroxide accepts a hydrogen ion from a water molecule?
(Multiple Choice)
4.8/5  (34)
(34)
What would the concentration of H3O+ be if the concentration of OH- was 1 × 10-1M? [H3O+] × [OH-] = Kw = 1 × 10-14
(Multiple Choice)
4.7/5  (33)
(33)
What would the concentration of OH- be if the concentration of H3O+ was 1 × 10-5M? [H3O+] × [OH-] = Kw = 1 × 10-14
(Multiple Choice)
4.9/5  (30)
(30)
In a battery,the following two oxidation-reduction reactions are taking place: rxn A: Zn + 2 OH- → ZnO + H2O + 2e-
Rxn B: 2 MnO2 + H2O + 2e- → Mn2O3 + 2 OH-
What is undergoing reduction?
(Multiple Choice)
4.9/5  (43)
(43)
According to the following reaction,which molecule is acting as a base? H3O+ + HSO4- → H2O + H2SO4
(Multiple Choice)
4.8/5  (41)
(41)
Qualitatively,what happens to the hydronium ion concentration if you increase the hydroxide ion concentration?
(Multiple Choice)
4.8/5  (30)
(30)
Chemical equations need to be balanced not only in terms of the number of atoms,but also by the charge.In other words,just as there should be the same number of atoms before and after the arrow of an equation,there should be the same charge.What set of coefficients is necessary to balance the following chemical equation? ________ + ________ Cl? ? ________ + ________
(Multiple Choice)
4.9/5  (30)
(30)
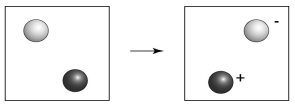 -In the above diagram,which atom is oxidized,the darker colored atom or the lighter colored atom?
-In the above diagram,which atom is oxidized,the darker colored atom or the lighter colored atom?
(Multiple Choice)
4.9/5  (37)
(37)
Why might an area with a large amount of limestone (CaCO3)be less susceptible to acid rain?
(Multiple Choice)
4.7/5  (29)
(29)
For the following acid-base reaction,identify what is formed in the space marked. HF + KOH ⇌ ???? + H2O
(Multiple Choice)
4.9/5  (20)
(20)
Upon ingestion,grain alcohol, O,is metabolized into acetaldehyde, O,which is a toxic substance causing headaches as well as joint pains typical of a "hangover." Is the grain alcohol oxidized or reduced as it transforms into acetaldehyde?
(Multiple Choice)
4.8/5  (39)
(39)
One of the products of combustion is water.Why doesn't this water extinguish the combustion?
(Multiple Choice)
4.9/5  (36)
(36)
Showing 161 - 177 of 177
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)