Exam 18: Two Classes of Chemical Reactions
Exam 1: Patterns of Motion and Equilibrium126 Questions
Exam 2: Newtons Laws of Motion127 Questions
Exam 3: Momentum and Energy181 Questions
Exam 4: Gravity, Projectiles, and Satellites147 Questions
Exam 5: Fluid Mechanics148 Questions
Exam 6: Thermal Energy and Thermodynamics113 Questions
Exam 7: Heat Transfer and Change of Phase158 Questions
Exam 8: Static and Current Electricity178 Questions
Exam 9: Magnetism and Electromagnetic Induction130 Questions
Exam 10: Waves and Sound146 Questions
Exam 11: Light163 Questions
Exam 12: Atoms and the Periodic Table137 Questions
Exam 13: The Atomic Nucleus and Radioactivity127 Questions
Exam 14: Elements of Chemistry69 Questions
Exam 15: How Atoms Bond and Molecules Attract150 Questions
Exam 16: Mixtures141 Questions
Exam 17: How Chemicals React120 Questions
Exam 18: Two Classes of Chemical Reactions177 Questions
Exam 19: Organic Compounds96 Questions
Exam 20: Rocks and Minerals169 Questions
Exam 21: Plate Tectonics and Earths Interior181 Questions
Exam 22: Shaping Earths Surface180 Questions
Exam 23: Geologic Time - Reading the Rock Record167 Questions
Exam 24: The Oceans, Atmosphere, and Climatic Effects188 Questions
Exam 25: Driving Forces of Weather166 Questions
Exam 26: The Solar System108 Questions
Exam 27: Stars and Galaxies107 Questions
Exam 28: The Structure of Space and Time73 Questions
Exam 29: Prologue: The Nature of Science22 Questions
Select questions type
How many electrons are gained or lost in the following half-reaction? Zn → Zn+2
(Multiple Choice)
4.9/5  (39)
(39)
If the pH of a solution is 10,what is the hydronium ion concentration?
(Multiple Choice)
4.7/5  (29)
(29)
Which of the following statements is untrue about oxidation and reduction processes?
(Multiple Choice)
4.9/5  (35)
(35)
Your car lights were left on while you were shopping,and now your car battery is dead.Has the pH of the battery fluid increased or decreased?
(Multiple Choice)
4.9/5  (33)
(33)
Hydrogen sulfide,H2S,burns in the presence of oxygen, ,to produce water, O,and sulfur dioxide,SO2.Through this reaction,is sulfur oxidized or reduced? 2 S + 3 ? 2 O + 2
(Multiple Choice)
4.9/5  (27)
(27)
Glucose, ,is a simple sugar that the body metabolizes into two molecules of pyruvic acid, .Is the glucose oxidized or reduced as it transforms into pyruvic acid?
(Multiple Choice)
4.8/5  (29)
(29)
In the reaction below,what does the symbol ⇌ mean? OH- + NH4+ ⇌ H2O + NH3
(Multiple Choice)
4.9/5  (33)
(33)
The amphoteric reaction between two water molecules is endothermic,which means that this reaction requires the input of energy in order to proceed: Energy + O + O ? + O The warmer the temperature of the water,the more thermal energy is available for this reaction,and the more hydronium and hydroxide ions are formed.Based on this information,should the value of be expected to increase,decrease,or stay the same with increasing temperature?
(Multiple Choice)
4.7/5  (32)
(32)
How is electrolysis different from what is going on chemically inside a battery?
(Multiple Choice)
4.9/5  (33)
(33)
How does connecting a metal like iron with a wire to a metal that is easier to oxidize like zinc help prevent corrosion of the iron?
(Multiple Choice)
4.7/5  (28)
(28)
In a battery,the following oxidation-reduction reactions are taking place: Mn2O3 + ZnO → 2 MnO2 + Zn
What is being formed at the anode?
(Multiple Choice)
4.8/5  (31)
(31)
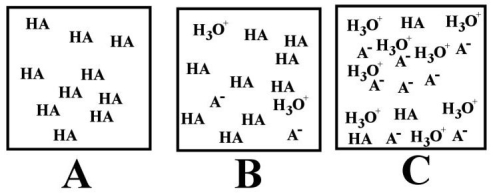 -Which of the above images represents a solution of the strongest acid (HA)?
-Which of the above images represents a solution of the strongest acid (HA)?
(Multiple Choice)
4.9/5  (31)
(31)
Which of the following compounds would least likely act as an acid?
(Multiple Choice)
4.8/5  (34)
(34)
What would be the concentration of hydronium ions in a solution that had a pH = -3? Why would such a solution be impossible to prepare?
(Multiple Choice)
4.9/5  (39)
(39)
Which of the following statements is not true about a neutralization reaction?
(Multiple Choice)
4.7/5  (29)
(29)
Identify the acid or base behavior of each substance in these reactions: HSO4- + O ? O + S
(Multiple Choice)
4.8/5  (35)
(35)
The oxidation of iron to rust is a problem structural engineers need to be concerned about,but the oxidation of aluminum to aluminum oxide is not so much of a problem.Why?
(Multiple Choice)
4.8/5  (33)
(33)
Which of the following species is the oxidizing agent? 2 CuBr → 2Cu + Br2
(Multiple Choice)
4.9/5  (23)
(23)
Showing 81 - 100 of 177
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)