Exam 16: Applications of Aqueous Equilibria
Exam 1: Chemical Tools: Experimentation and Measurement154 Questions
Exam 2: Atoms, molecules, and Ions275 Questions
Exam 3: Mass Relationships in Chemical Reactions159 Questions
Exam 4: Reactions in Aqueous Solutions211 Questions
Exam 5: Periodicity and the Electronic Structure of Atoms168 Questions
Exam 6: Ionic Compounds: Periodic Trends and Bonding138 Questions
Exam 7: Covalent Bonding and Electron-Dot Structures92 Questions
Exam 8: Covalent Compounds: Bonding Theories and Molecular Structure205 Questions
Exam 9: Thermochemistry: Chemical Energy170 Questions
Exam 10: Gases: Their Properties and Behavior188 Questions
Exam 11: Liquids, solids, and Phase Changes141 Questions
Exam 12: Solutions and Their Properties198 Questions
Exam 13: Chemical Kinetics190 Questions
Exam 14: Chemical Equilibrium171 Questions
Exam 15: Aqueous Equilibria: Acids and Bases231 Questions
Exam 16: Applications of Aqueous Equilibria201 Questions
Exam 17: Thermodynamics: Entropy, free Energy, and Equilibrium150 Questions
Exam 18: Electrochemistry212 Questions
Exam 19: Nuclear Chemistry176 Questions
Exam 20: Transition Elements and Coordination Chemistry190 Questions
Exam 21: Metals and Solid-State Materials154 Questions
Exam 22: The Main-Group Elements294 Questions
Exam 23: Connections to Organic and Biological Chemistry290 Questions
Select questions type
What is the approximate value of the equilibrium constant,Kn,for the neutralization of nitrous acid with ammonia,shown in the equation below? The Ka for HNO2 is 4.5 × 10-4 and the Kb for NH3 is 1.8 × 10-5. HNO2(aq)+ NH3(aq)⇌ NH4NO2(aq)
(Multiple Choice)
4.8/5  (38)
(38)
What is the approximate value of the equilibrium constant,Kn,for the neutralization of acetic acid with potassium hydroxide,shown in the equation below? The Ka for acetic acid is 1.8 × 10-5. CH3CO2H(aq)+ KOH(aq)⇌ H2O(l)+ NaCH3CO2(aq)
(Multiple Choice)
4.8/5  (44)
(44)
What volume of 5.00 × 10-3 M HNO3 is needed to titrate 100.00 mL of 5.00 × 10-3 M Ca(OH)2 to the equivalence point?
(Multiple Choice)
4.8/5  (32)
(32)
The balanced net ionic equation for the neutralization reaction involving equal molar amounts of
HCl and CH3CH2NH2 is ________.
(Essay)
4.9/5  (37)
(37)
What is the pH of the solution formed when 50 mL of 0.250 M NaOH is added to 50 mL of 0.120 M HCl?
(Short Answer)
4.9/5  (37)
(37)
The following pictures represent solutions at various points in the titration of a weak acid HA with aqueous KOH.Unshaded spheres represent H atoms,black spheres represent oxygen atoms,and shaded spheres represent A- ions.(K+,H3O+ initially present,OH- initially present and solvent water molecules have been omitted for clarity).  -Which picture represents the solution before the equivalence point?
-Which picture represents the solution before the equivalence point?
(Multiple Choice)
4.9/5  (41)
(41)
What is the resulting pH when 0.005 moles of KOH is added to 0.100 L of a buffer solution that is 0.100 M in H2PO4- and 0.100 M HPO42- and the Ka2 = 6.2 × 10-8?
(Multiple Choice)
4.8/5  (33)
(33)
What is the common ion in a solution prepared by mixing 0.55 M LiCH3CO2 with 0.10 M CH3CO2H?
(Multiple Choice)
4.8/5  (38)
(38)
What is the pH of a solution prepared by mixing 25.00 mL of 0.10 M methylamine,CH3NH2,with 25.00 mL of 0.10 M methylammonium chloride,CH3NH3Cl? Assume that the volume of the solutions are additive and that Kb = 3.70 × 10-4 for methylamine.
(Multiple Choice)
4.8/5  (29)
(29)
The following plot shows a titration curve for the titration of 1.00 L of 1.00 M diprotic acid H2A with NaOH.  -Which point a-d represents pKa2?
-Which point a-d represents pKa2?
(Multiple Choice)
4.8/5  (44)
(44)
What is the Ka of the amino acid glutamine if it is 33.0% dissociated at pH = 8.82?
(Short Answer)
4.9/5  (37)
(37)
What is the pH of 1 L of 0.30 M TRIS,0.60 M TRISH+ buffer to which one has added 5.0 mL of 12 M HCl? The Kb for the TRIS/TRISH+ is 1.2 × 10-6.
(Multiple Choice)
4.8/5  (39)
(39)
What is the molar solubility of AgCl in 0.10 M NH3? Ksp for AgCl is 1.8 × 10-10 and the Kf for Ag(NH3)2+ is 1.7 × 107.
(Multiple Choice)
4.8/5  (45)
(45)
What is the pH of a solution prepared by mixing 50.00 mL of 0.10 M NH3 with 20.00 mL of 0.10 M NH4Cl? Assume that the volume of the solutions are additive and that Kb = 1.8 × 10-5 for NH3.
(Multiple Choice)
4.8/5  (35)
(35)
What is the molar solubility of Mg(OH)2 in a basic solution with a pH of 12.00? Ksp for Mg(OH)2 is 5.6 × 10-12.
(Multiple Choice)
4.9/5  (36)
(36)
What is the molar solubility of AgCl in 0.50 M NaCN if the colorless complex ion Ag(CN)2- forms? Ksp for AgCl is 1.8 × 10-10 and Kf for Ag(CN)2- is 1.0 × 1021.
(Multiple Choice)
4.8/5  (41)
(41)
The following pictures represent solutions that contain a weak acid HA and/or its potassium salt KA.Unshaded spheres represent H atoms and shaded spheres represent A- ions.(K+,H3O+,OH-,and solvent H2O molecules have been omitted for clarity. ) 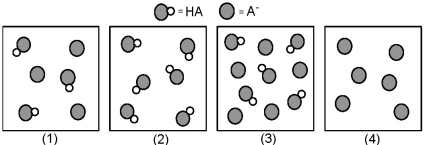 -Which solution has the lowest pH?
-Which solution has the lowest pH?
(Multiple Choice)
4.8/5  (31)
(31)
Which is a net ionic equation for the neutralization of a strong acid with a strong base?
(Multiple Choice)
4.8/5  (35)
(35)
The following plot shows two titration curves,each representing the titration of 50.00 mL of 0.100 M acid with 0.100 M NaOH.  -Which points a-d represent the half-equivalence point and the equivalence point,respectively,for the titration of a weak acid?
-Which points a-d represent the half-equivalence point and the equivalence point,respectively,for the titration of a weak acid?
(Multiple Choice)
4.7/5  (35)
(35)
The following pictures represent solutions of CuS,which may also contain ions other than Cu2+ and S2- which are not shown.Gray spheres represent Cu2+ ions and dotted spheres represent S2- ions.  -If solution (1)is a saturated solution of CuS,which of solutions (2)-(4)are saturated?
-If solution (1)is a saturated solution of CuS,which of solutions (2)-(4)are saturated?
(Multiple Choice)
5.0/5  (30)
(30)
Showing 141 - 160 of 201
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)