Exam 16: Applications of Aqueous Equilibria
Exam 1: Chemical Tools: Experimentation and Measurement154 Questions
Exam 2: Atoms, molecules, and Ions275 Questions
Exam 3: Mass Relationships in Chemical Reactions159 Questions
Exam 4: Reactions in Aqueous Solutions211 Questions
Exam 5: Periodicity and the Electronic Structure of Atoms168 Questions
Exam 6: Ionic Compounds: Periodic Trends and Bonding138 Questions
Exam 7: Covalent Bonding and Electron-Dot Structures92 Questions
Exam 8: Covalent Compounds: Bonding Theories and Molecular Structure205 Questions
Exam 9: Thermochemistry: Chemical Energy170 Questions
Exam 10: Gases: Their Properties and Behavior188 Questions
Exam 11: Liquids, solids, and Phase Changes141 Questions
Exam 12: Solutions and Their Properties198 Questions
Exam 13: Chemical Kinetics190 Questions
Exam 14: Chemical Equilibrium171 Questions
Exam 15: Aqueous Equilibria: Acids and Bases231 Questions
Exam 16: Applications of Aqueous Equilibria201 Questions
Exam 17: Thermodynamics: Entropy, free Energy, and Equilibrium150 Questions
Exam 18: Electrochemistry212 Questions
Exam 19: Nuclear Chemistry176 Questions
Exam 20: Transition Elements and Coordination Chemistry190 Questions
Exam 21: Metals and Solid-State Materials154 Questions
Exam 22: The Main-Group Elements294 Questions
Exam 23: Connections to Organic and Biological Chemistry290 Questions
Select questions type
In which of the following solutions would solid AgCl be expected to be the least soluble at 25°C?
(Multiple Choice)
4.9/5  (30)
(30)
Formic acid (HCO2H,Ka = 1.8 × 10-4)is the principal component in the venom of stinging ants.What is the molarity of a formic acid solution if 25.00 mL of the formic acid solution requires 29.80 mL of 0.0567 M NaOH to reach the equivalence point?
(Multiple Choice)
4.9/5  (28)
(28)
When equal molar amounts of the following sets of compounds are mixed in water,which will not form a buffer solution?
(Multiple Choice)
4.9/5  (34)
(34)
Precipitation of an ionic compound will occur upon mixing of desired reagents if the initial ion product is
(Multiple Choice)
4.8/5  (35)
(35)
The following pictures represent solutions that contain a weak acid HA (pKa = 5.0)and its potassium salt KA.Unshaded spheres represent H atoms and shaded spheres represent A- ions.(K+,H3O+,OH-,and solvent H2O molecules have been omitted for clarity. ) 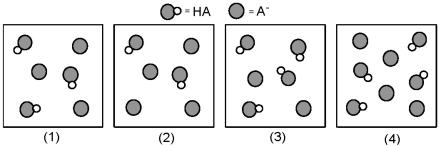 -For which of these solutions is pH = pKa?
-For which of these solutions is pH = pKa?
(Multiple Choice)
4.8/5  (38)
(38)
TRIS {(HOCH2)3CNH2} is one of the most common buffers used in biochemistry.A solution is prepared by adding enough TRIS and 12 M HCl(aq)to give 1.00 L of solution with [TRIS] = 0.30 M and [TRISH+] = 0.60 M.What is the pH of this buffered system if the pKb is 5.92?
(Multiple Choice)
4.9/5  (38)
(38)
What is the pH of a solution made by mixing 30.00 mL of 0.10 M HCl with 40.00 mL of 0.10 M KOH? Assume that the volumes of the solutions are additive.
(Multiple Choice)
4.8/5  (33)
(33)
In which of the following solutions would solid PbBr2 be expected to be the least soluble at 25°C?
(Multiple Choice)
4.8/5  (45)
(45)
Which is a net ionic equation for the neutralization of a weak acid with a strong base?
(Multiple Choice)
4.8/5  (30)
(30)
Use the graphs below to answer the following questions.  -What is the characteristic pH-titrant curve for the titration of a strong acid by a strong base?
-What is the characteristic pH-titrant curve for the titration of a strong acid by a strong base?
(Multiple Choice)
4.8/5  (31)
(31)
The following pictures represent solutions of AgCl,which may also contain ions other than Ag+ and Cl- which are not shown.Gray spheres represent Ag+ ions and dotted spheres represent Cl- ions.  -If solution (1)is a saturated solution of AgCl,which of solutions (1)-(4)represents the solution after a small amount of HCl is added and equilibrium is restored?
-If solution (1)is a saturated solution of AgCl,which of solutions (1)-(4)represents the solution after a small amount of HCl is added and equilibrium is restored?
(Multiple Choice)
4.9/5  (38)
(38)
The following plot shows two titration curves,each representing the titration of 50.00 mL of 0.100 M acid with 0.100 M NaOH.  -Which point a-d represents a buffer region?
-Which point a-d represents a buffer region?
(Multiple Choice)
4.9/5  (38)
(38)
At what pH is the amino acid glycine with a Ka of 2.51 × 10-10 sixty-six (66%)percent dissociated?
(Multiple Choice)
4.8/5  (38)
(38)
The following pictures represent solutions of CuS,which may also contain ions other than Cu2+ and S2- which are not shown.Gray spheres represent Cu2+ ions and dotted spheres represent S2- ions.  -If solution (1)is a saturated solution of CuS,which of solutions (2)-(4)are supersaturated?
-If solution (1)is a saturated solution of CuS,which of solutions (2)-(4)are supersaturated?
(Multiple Choice)
4.7/5  (32)
(32)
Addition of 0.0125 mol HCl to 150 mL of a 0.150 M formic acid/0.100 M sodium formate buffer results in a pH = ________.The Ka of formic acid is 1.8 × 10-4.
(Short Answer)
4.9/5  (37)
(37)
What is the pH at the equivalence point of a weak acid-strong base titration?
(Multiple Choice)
4.8/5  (44)
(44)
Formic acid (HCO2H,Ka = 1.8 × 10-4)is the principal component in the venom of stinging ants.What is the molarity of a formic acid solution if 25.00 mL of the formic acid solution requires 44.80 mL of 0.0567 M NaOH to reach the equivalence point?
(Multiple Choice)
4.8/5  (36)
(36)
What is the percent dissociation of acetic acid if the solution has a pH = 4.74 and a pKa = 4.74?
(Multiple Choice)
4.7/5  (36)
(36)
The following plot shows a titration curve for the titration of 1.00 L of 1.00 M diprotic acid H2A with NaOH.  -A buffer region is indicated by which point(s)a-d?
-A buffer region is indicated by which point(s)a-d?
(Multiple Choice)
4.8/5  (48)
(48)
The following pictures represent solutions that contain a weak acid HA (pKa = 5.0)and its potassium salt KA.Unshaded spheres represent H atoms and shaded spheres represent A- ions.(K+,H3O+,OH-,and solvent H2O molecules have been omitted for clarity. )  -Which solution has the greatest buffer capacity?
-Which solution has the greatest buffer capacity?
(Multiple Choice)
4.9/5  (43)
(43)
Showing 41 - 60 of 201
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)