Exam 16: Applications of Aqueous Equilibria
Exam 1: Chemical Tools: Experimentation and Measurement154 Questions
Exam 2: Atoms, molecules, and Ions275 Questions
Exam 3: Mass Relationships in Chemical Reactions159 Questions
Exam 4: Reactions in Aqueous Solutions211 Questions
Exam 5: Periodicity and the Electronic Structure of Atoms168 Questions
Exam 6: Ionic Compounds: Periodic Trends and Bonding138 Questions
Exam 7: Covalent Bonding and Electron-Dot Structures92 Questions
Exam 8: Covalent Compounds: Bonding Theories and Molecular Structure205 Questions
Exam 9: Thermochemistry: Chemical Energy170 Questions
Exam 10: Gases: Their Properties and Behavior188 Questions
Exam 11: Liquids, solids, and Phase Changes141 Questions
Exam 12: Solutions and Their Properties198 Questions
Exam 13: Chemical Kinetics190 Questions
Exam 14: Chemical Equilibrium171 Questions
Exam 15: Aqueous Equilibria: Acids and Bases231 Questions
Exam 16: Applications of Aqueous Equilibria201 Questions
Exam 17: Thermodynamics: Entropy, free Energy, and Equilibrium150 Questions
Exam 18: Electrochemistry212 Questions
Exam 19: Nuclear Chemistry176 Questions
Exam 20: Transition Elements and Coordination Chemistry190 Questions
Exam 21: Metals and Solid-State Materials154 Questions
Exam 22: The Main-Group Elements294 Questions
Exam 23: Connections to Organic and Biological Chemistry290 Questions
Select questions type
What is the pH of a solution made by mixing 20.00 mL of 0.100 M HCl with 40.00 mL of 0.100 M KOH? Assume that the volumes of the solutions are additive.
(Multiple Choice)
4.9/5  (35)
(35)
The following pictures represent solutions at various stages in the titration of a weak diprotic acid H2A with aqueous KOH.Unshaded spheres represent H atoms,black spheres represent oxygen atoms,and shaded spheres represent A2- ions.(K+,H3O+ initially present,OH- initially present and solvent water molecules have been omitted for clarity). 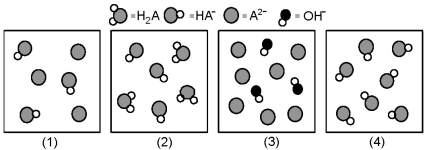 -Which picture represents the system halfway to the first equivalence point?
-Which picture represents the system halfway to the first equivalence point?
(Multiple Choice)
4.9/5  (35)
(35)
The following pictures represent solutions that contain a weak acid HA (pKa = 5.0)and its potassium salt KA.Unshaded spheres represent H atoms,black spheres represent oxygen atoms,and shaded spheres represent A- ions.(K+,H3O+ initially present,OH- initially present and solvent water molecules have been omitted for clarity. )  -Which picture represents the equilibrium state of the solution after addition of one OH- ion to the solution shown in picture (1)?
-Which picture represents the equilibrium state of the solution after addition of one OH- ion to the solution shown in picture (1)?
(Multiple Choice)
4.7/5  (48)
(48)
The artist's pigment cadmium yellow,CdS,has a water solubility of 0.13 g/L.The solubility product of CdS,Ksp = ________.
(Short Answer)
4.7/5  (27)
(27)
Which of the following titrations result in an acidic solution at the equivalence point?
(Multiple Choice)
4.9/5  (33)
(33)
What is the hydronium ion concentration in a solution prepared by mixing 50.00 mL of 0.10 M HCN with 50.00 mL of  NaCN? Assume that the volumes of the solutions are additive and that Ka = 4.9 × 10-10 for HCN.
NaCN? Assume that the volumes of the solutions are additive and that Ka = 4.9 × 10-10 for HCN.
(Multiple Choice)
4.9/5  (40)
(40)
Which of these neutralization reactions has a pH < 7 when equal molar amounts of acid and base are mixed?
(Multiple Choice)
4.9/5  (39)
(39)
The following pictures represent solutions at various points in the titration of a weak acid HA with aqueous KOH.Unshaded spheres represent H atoms,black spheres represent oxygen atoms,and shaded spheres represent A- ions.(K+,H3O+ initially present,OH- initially present and solvent water molecules have been omitted for clarity).  -Which picture represents the solution at the equivalence point?
-Which picture represents the solution at the equivalence point?
(Multiple Choice)
4.9/5  (35)
(35)
What is the hydronium ion concentration in a solution prepared by mixing 50.00 mL of 0.10 M HCN with 50.00 mL of  NaCN? Assume that the volumes of the solutions are additive and that Ka = 4.9 × 10-10 for HCN.
NaCN? Assume that the volumes of the solutions are additive and that Ka = 4.9 × 10-10 for HCN.
(Multiple Choice)
4.8/5  (39)
(39)
The neutralization constant Kn for the neutralization of penicillin V (C16H18N2O5S)and erythromycin (C37H67NO13)is 1.3 × 106.The acid dissociation constant Ka for penicillin V is 2.0 × 10-3.What is the base dissociation constant Kb for erythromycin?
(Short Answer)
4.7/5  (25)
(25)
What is the pH at the equivalence point of a weak base-strong acid titration if 20.00 mL of NaOCl requires 28.30 mL of 0.20 M HCl? Ka = 3.0 × 10-8 for HOCl.
(Multiple Choice)
4.7/5  (35)
(35)
Identify the following solutions as acidic,basic,or inert.
NaNO2 _______
NaNO3 ________
C5H5NHClO4 ________
(Short Answer)
4.8/5  (37)
(37)
The following plot shows a titration curve for the titration of 1.00 L of 1.00 M diprotic acid H2A with NaOH.  -Which point a-d represents the second equivalence point?
-Which point a-d represents the second equivalence point?
(Multiple Choice)
4.9/5  (39)
(39)
A buffer prepared by mixing equal moles of an acid having Ka = 4.5 × 10-4 and a salt of its conjugate base has a pH = ________.
(Short Answer)
4.8/5  (36)
(36)
The dissociation equilibrium constants for the protonated form of alanine (a diprotic amino acid,H2X+)are Ka1 = 4.6 × 10-3 and Ka2 = 2.0 × 10-10.What is the pH of 50.00 mL of a 0.0500 M solution of alanine after 25.00 mL of 0.100 M NaOH has been added?
(Multiple Choice)
4.8/5  (29)
(29)
What is the molar solubility of AgCl in 0.10 M NaCN if the colorless complex ion Ag(CN)2- forms? Ksp for AgCl is 1.8 × 10-10 and Kf for Ag(CN)2- is 1.0 × 1021.
(Multiple Choice)
4.8/5  (36)
(36)
The following pictures represent solutions at various stages in the titration of a weak diprotic acid H2A with aqueous KOH.Unshaded spheres represent H atoms,black spheres represent oxygen atoms,and shaded spheres represent A2- ions.(K+,H3O+ initially present,OH- initially present and solvent water molecules have been omitted for clarity).  -Which picture represents the system halfway between the first and second equivalence points?
-Which picture represents the system halfway between the first and second equivalence points?
(Multiple Choice)
4.9/5  (38)
(38)
What is the pH of a solution prepared by mixing 25.00 mL of 0.10 M CH3CO2H with 25.00 mL of 0.050 M CH3CO2Na? Assume that the volume of the solutions are additive and that Ka = 1.8 × 10-5 for CH3CO2H.
(Multiple Choice)
4.9/5  (30)
(30)
The following pictures represent solutions of CaCO3,which may also contain ions other than Ca2+ and CO32- which are not shown.Gray spheres represent Ca2+ ions and unshaded spheres represent CO32- ions.  -If solution (1)is a saturated solution of CaCO3,which of solutions (1)-(4)represents the solution after a small amount of K2CO3 is added and equilibrium is restored?
-If solution (1)is a saturated solution of CaCO3,which of solutions (1)-(4)represents the solution after a small amount of K2CO3 is added and equilibrium is restored?
(Multiple Choice)
4.9/5  (37)
(37)
Showing 181 - 200 of 201
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)