Exam 6: Reactions
Exam 1: Atoms202 Questions
Exam 2: Molecules181 Questions
Exam 3: States of Matter287 Questions
Exam 4: Thermodynamics192 Questions
Exam 5: Equilibrium191 Questions
Exam 6: Reactions282 Questions
Exam 7: Kinetics87 Questions
Exam 8: The Main-Group Elements191 Questions
Exam 9: The D-Block Elements95 Questions
Exam 10: Nuclear Chemistry96 Questions
Exam 11: Organic Chemistry193 Questions
Select questions type
When sulfur trioxide dissolves in water, sulfuric acid is produced.An intermediate in the reaction is H2O-SO3.In the reaction of the intermediate to produce sulfuric acid,
(Multiple Choice)
4.7/5  (39)
(39)
A solution is prepared by mixing equal volumes of 0.40 M HF(aq)with 0.20 M KOH(aq).This solution is a buffer.
(True/False)
4.9/5  (31)
(31)
For pyridine, pKb = 8.75.What is the pH of an aqueous buffer solution that is 0.300 M C5H5N(aq)And 0.500 MC5H5NHCl(aq)?
(Multiple Choice)
4.8/5  (31)
(31)
If you wish to increase the solubility of silver benzoate, a preservative,
You would
(Multiple Choice)
4.9/5  (33)
(33)
If E
for the disproportionation of Cu+(aq) to Cu2+(aq)And Cu(s)Is +0.18 V at 25 C,Calculate the equilibrium constant for the reaction.
(Multiple Choice)
4.8/5  (40)
(40)
Is a 0.1 M aqueous solution of HPO42- acidic, basic,or neutral? Prove your answer using the appropriate equilibrium constants.
(Essay)
4.8/5  (50)
(50)
In a solution that is labeled "0.10 M H3PO4(aq)," [H3O+] = 0.024 M.Match the species below with their concentrations.
6.2\times1 8.0\times1 5.4\times1 2.4\times1
(Essay)
4.9/5  (44)
(44)
Consider the following cell:
Zn(s)|Zn2+(aq,0.10 M)m Cu2+(aq,0.10 M)|Cu(s)
At equilibrium,what is the concentration of Zn2+(aq)?
(Short Answer)
4.7/5  (37)
(37)
Given: Cr(OH)3(s) CrO42-(aq),Basic solution.How many electrons appear in the balanced half-reaction?
(Multiple Choice)
4.9/5  (36)
(36)
Of the following five ions or molecules, which is the strongest reducing agent?
(Multiple Choice)
4.7/5  (40)
(40)
What is the relationship between the solubility in water, s,And Ksp for the ionic solid Ca3(PO4)2?
(Multiple Choice)
4.9/5  (38)
(38)
The titration curve for the titration of 0.100 M H2SO3(aq)
with 0.100 M KOH(aq) is given below. 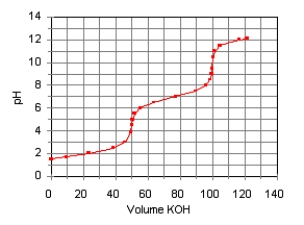 Estimate pKa1 and pKa2 of H2SO3.
Estimate pKa1 and pKa2 of H2SO3.
(Short Answer)
4.9/5  (41)
(41)
If the standard potential for Tl3+(aq)/Tl+(aq) is 1.21 V and the standard potential for Tl+(aq)/Tl(s)Is -0.34 V,Calculate the standard potential for
Tl3+(aq)+ 3e- Tl(s).
(Multiple Choice)
4.8/5  (40)
(40)
When equilibrium is reached in an electrochemical cell,the voltage reaches its maximum value.
(True/False)
4.9/5  (37)
(37)
What reaction occurs at the cathode in the following cell diagram?
Cd(s|CdSO4(aq)|Hg2SO4|Hg(l)
(Short Answer)
4.8/5  (38)
(38)
A cell that uses bromine to oxidize chloride ion under standard conditions at 298 K has a positive potential.
(True/False)
4.9/5  (41)
(41)
Calculate the ratio of the molarities of HPO42- and H2PO4- ions required to achieve buffering at pH = 7.00 .For H3PO4,PKa1 = 2.12,PKa2 = 7.21,And pKa3 = 12.68.
(Multiple Choice)
4.8/5  (38)
(38)
How many moles of Cl2(g) are produced by the electrolysis of concentrated sodium chloride if 2.00 A are passed through the solution for 4.00 hours? The equation for this process,The "chloralkali" process,Is
2NaCl(aq)+ 2H2O(l) 2NaOH(aq)+ H2(g)+ Cl2(g)
(Multiple Choice)
4.8/5  (32)
(32)
What is the equilibrium constant for the reaction
HCN(aq)+ OH-(aq)
CN-(aq)+ H2O(l)
(Multiple Choice)
4.9/5  (41)
(41)
Showing 221 - 240 of 282
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)