Exam 6: Reactions
Exam 1: Atoms202 Questions
Exam 2: Molecules181 Questions
Exam 3: States of Matter287 Questions
Exam 4: Thermodynamics192 Questions
Exam 5: Equilibrium191 Questions
Exam 6: Reactions282 Questions
Exam 7: Kinetics87 Questions
Exam 8: The Main-Group Elements191 Questions
Exam 9: The D-Block Elements95 Questions
Exam 10: Nuclear Chemistry96 Questions
Exam 11: Organic Chemistry193 Questions
Select questions type
Choose the effective pH range of a pyridine/pyridinium chloride buffer? For pyridine, the value of Kb is 1.8 * 10-9.
(Multiple Choice)
4.9/5  (37)
(37)
What is E for the half-reaction below?
H2(g,1.00 atm) 2H+(aq,1.00 M)+ 2e-
(Short Answer)
4.9/5  (37)
(37)
Consider the following cell:
Pt|H2(g,1 atm)|H+(aq,? M)M Ag+(aq,1.0 M)|Ag(s)
If the voltage of this cell is 1.04 V at25 C and the standard potential of the Ag+/Ag couple is +0.80 V,Calculate the hydrogen ion concentration in the anode compartment.
(Multiple Choice)
4.9/5  (40)
(40)
Which of the following indicators would be most suitable for the titration of 0.10 M lactic acid with 0.10 M KOH(aq)? For lactic acid, pKa = 3.08.
(Multiple Choice)
4.8/5  (45)
(45)
Use the following to answer questions 55-58: 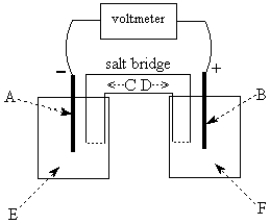 -The galvanic cell shown above uses the half-cells Pb2+/Pb and Zn2+/Zn, and a salt bridge containing KCl(aq).The voltmeter gives a positive voltage reading.Identify A and write the half-reaction that occurs in that compartment.Does the size of the electrode A increase or decrease during operation of the cell? What is the voltmeter reading?
-The galvanic cell shown above uses the half-cells Pb2+/Pb and Zn2+/Zn, and a salt bridge containing KCl(aq).The voltmeter gives a positive voltage reading.Identify A and write the half-reaction that occurs in that compartment.Does the size of the electrode A increase or decrease during operation of the cell? What is the voltmeter reading?
(Essay)
4.8/5  (36)
(36)
The following 0.1 M aqueous solutions are arranged in order of increasing pH, with the highest pH on the far right.
Which one of the following 0.10 M aqueous solutions should be placed in the empty box?
(Multiple Choice)
4.9/5  (44)
(44)
Write the charge balance equation for a dilute aqueous solution of HI.
(Multiple Choice)
4.9/5  (37)
(37)
When the Ag(s)|AgCl(s|Cl-(aq) electrode acts as a cathode,
The reaction is
(Multiple Choice)
4.9/5  (37)
(37)
The solubility of silver bromide is greater in 0.10 M NaBr than in pure water.
(True/False)
4.8/5  (41)
(41)
If a small amount of a strong acid is added to buffer made up of a weak acid, HA,And the sodium salt of its conjugate base,NaA,The pH of the buffer solution does not change appreciably because
(Multiple Choice)
4.9/5  (41)
(41)
The Ka of phenol is 1.3 * 10-10 .For a solution labeled "1.0 * 10-3 M aqueous phenol,"
(Multiple Choice)
4.9/5  (39)
(39)
A buffer solution contains 0.75 mol KH2PO4 and 0.75 mol K2HPO4 .
What is the pH after 0.10 mol KOH is added to 1.00 L of this buffer? The pKa of H2PO4? is 7.21.
(Multiple Choice)
4.9/5  (39)
(39)
Use the following to answer questions 55-58:  -The galvanic cell shown above uses the half-cells Pb2+/Pb and Zn2+/Zn,and a salt bridge containing KCl(aq).The voltmeter gives a positive voltage reading.Theelectrode B could be inert platinum metal or lead.
-The galvanic cell shown above uses the half-cells Pb2+/Pb and Zn2+/Zn,and a salt bridge containing KCl(aq).The voltmeter gives a positive voltage reading.Theelectrode B could be inert platinum metal or lead.
(True/False)
4.7/5  (49)
(49)
What is the pH of an aqueous solution that is 0.011 M HF (Ka = 3.5 * 10-4) and 0.015 M NaF?
(Multiple Choice)
4.8/5  (32)
(32)
If 8686 C of charge is passed through molten magnesium chloride, calculate the number of moles of Mg(l)
Produced.
(Multiple Choice)
4.7/5  (46)
(46)
If pKa1 and pKa2 for H2S are 6.88 and 14.15, respectively,Calculate the equilibrium constant for the reaction below:
H2S(aq)+ 2H2O(l) 2H3O+(aq)+ S2-(aq)
(Multiple Choice)
4.8/5  (35)
(35)
Showing 261 - 280 of 282
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)