Exam 6: Reactions
Exam 1: Atoms202 Questions
Exam 2: Molecules181 Questions
Exam 3: States of Matter287 Questions
Exam 4: Thermodynamics192 Questions
Exam 5: Equilibrium191 Questions
Exam 6: Reactions282 Questions
Exam 7: Kinetics87 Questions
Exam 8: The Main-Group Elements191 Questions
Exam 9: The D-Block Elements95 Questions
Exam 10: Nuclear Chemistry96 Questions
Exam 11: Organic Chemistry193 Questions
Select questions type
Consider the titration of 15.0 mL of 0.200 M H3PO4(aq) with 0.200 M NaOH(aq).What is/are the major species in solution after the addition of 30.0 mL of base?
(Multiple Choice)
4.7/5  (36)
(36)
Of the following metals, which metal would be suitable to provide an iron bridge with cathodic protection from corrosion?
(Multiple Choice)
4.8/5  (37)
(37)
The standard voltage of the cell
Ag(s)|AgBr(s)|Br-(aq)M Ag+(aq)|Ag(s)
Is +0.73 V at 25 C.Calculate the Ksp for AgBr.
(Multiple Choice)
4.9/5  (43)
(43)
Calculate E for the half-reaction below.
2H+(aq,1.00 * 10-5 M)+ 2e- H2(g,1.00 atm)
(Multiple Choice)
4.8/5  (38)
(38)
The pH of 0.800 M aqueous benzenesulfonic acid is 0.51 .What is the value of Ka for benzenesulfonic acid?
(Multiple Choice)
4.9/5  (39)
(39)
Use the following to answer questions 55-58: 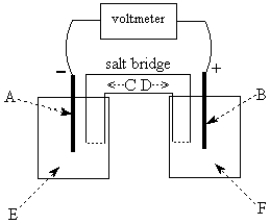 -The galvanic cell shown above uses the half-cells Mg2+/Mg and Zn2+/Zn, and a salt bridge containing KCl(aq).The voltmeter gives a positive voltage reading.Identify A and write the half-reaction that occurs in that compartment.Does the size of the electrode A increase or decrease during operation of the cell? What is the voltmeter reading?
-The galvanic cell shown above uses the half-cells Mg2+/Mg and Zn2+/Zn, and a salt bridge containing KCl(aq).The voltmeter gives a positive voltage reading.Identify A and write the half-reaction that occurs in that compartment.Does the size of the electrode A increase or decrease during operation of the cell? What is the voltmeter reading?
(Essay)
4.9/5  (36)
(36)
What is the [H+] for a solution labeled "0.0500 M H2SO3(aq)" if pKa1 = 1.81, and pKa2 = 6.91?
(Multiple Choice)
4.9/5  (33)
(33)
The following compounds are available as 0.10 M aqueous solutions. A) pyridine B) C) D) E) F) phenol G) triethylamine H) I)
Which two solutions could be used to prepare a buffer with a pH of ~ 9? More than one answer may be possible.
(Short Answer)
4.9/5  (34)
(34)
Given: Zn(s) + OH-(aq)+ H2O(l)+ NO3-(aq Zn(OH)42-(aq)+ NH3(g)
If the coefficient of NO3- in the balanced equation is 1,How many electrons are transferred in the reaction?
(Multiple Choice)
4.9/5  (38)
(38)
The weak base, B,Has Kb = 3.1 × 10-4.The pH of a solution in which [B] = [BH+] is
(Multiple Choice)
4.8/5  (25)
(25)
The titration curve for the titration of 0.100 M H2SO3(aq) with 0.100 M KOH(aq) Is given below. 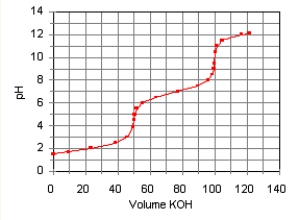 The major species in solution after 75 mL of KOH(aq) Has been added are
The major species in solution after 75 mL of KOH(aq) Has been added are
(Multiple Choice)
4.9/5  (41)
(41)
Which of the following mixtures gives a buffer with a pH greater than 7.0? For HCNO, Ka = 2.2 * 10-4 and for NH3,Kb = 1.8 * 10-5.
(Multiple Choice)
4.7/5  (31)
(31)
In the determination of iron in vitamins, Fe2+ is titrated with permanganate, MnO4-,In acidic solution.The products of the reaction are Fe3+ and Mn2+.In the balanced equation,The number of electrons transferred is
(Multiple Choice)
4.8/5  (42)
(42)
Assuming no volume change on mixing,what mass of ammonium chloride should be added to 1.00 L of 0.250 M NH3(aq)to produce a buffer of pH 10.10? The molar mass of ammonium chloride is 53.49 g/mol.
(Short Answer)
4.8/5  (29)
(29)
The pH of 0.010 M H3PO4(aq) is 2.24; estimate the concentration of PO43- in the solution.For H3PO4,The values of Ka1,Ka2,And Ka3 are 7.6 * 10-3,
6.2 * 10-8,And 2.1 * 10-13,Respectively.
(Multiple Choice)
4.8/5  (31)
(31)
What is the pH of an aqueous solution that is 0.018 M C6H5NH2 (Kb = 4.3 * 10-10) and 0.12 M C6H5NH3Cl?
(Multiple Choice)
4.8/5  (40)
(40)
Use the following:
Pb(s)|PbSO4(s)|SO42-(aq,0.60 M)M H+(aq,0.70 M)|H2(g,192.5 kPa)|Pt
In this cell,If E is 0.36 V at 25 C,What is the Nernst equation for the cell at this temperature?
(Multiple Choice)
4.7/5  (36)
(36)
The fractional composition diagram for the amino acid alanine is shown below. 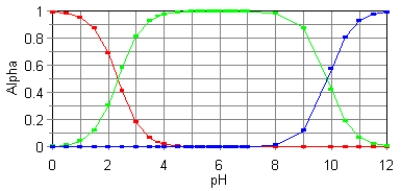 What do the two points represent where alpha is 0.5?
What do the two points represent where alpha is 0.5?
(Short Answer)
4.8/5  (38)
(38)
Showing 181 - 200 of 282
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)