Exam 6: Reactions
Exam 1: Atoms202 Questions
Exam 2: Molecules181 Questions
Exam 3: States of Matter287 Questions
Exam 4: Thermodynamics192 Questions
Exam 5: Equilibrium191 Questions
Exam 6: Reactions282 Questions
Exam 7: Kinetics87 Questions
Exam 8: The Main-Group Elements191 Questions
Exam 9: The D-Block Elements95 Questions
Exam 10: Nuclear Chemistry96 Questions
Exam 11: Organic Chemistry193 Questions
Select questions type
For NH3, pKb = 4.74.What is the pH of an aqueous buffer solution that is 0.050 M NH3(aq)And 0.20 M NH4Cl(aq)?
(Multiple Choice)
4.9/5  (47)
(47)
In a working electrochemical cell (+ cell voltage),the electrons flow from the anode through the external circuit to the cathode.
(True/False)
4.7/5  (37)
(37)
The following compounds are available as 0.10 M aqueous solutions. A) pyridine B) C) D) E) F) phenol G) triethylamine H) I)
Which two solutions could be used to prepare a buffer with a pH of ~ 2.5?
(Short Answer)
4.9/5  (41)
(41)
For a 0.10 M solution of a weak acid, HA,With pKa = 10,Which of the following is true?
(Multiple Choice)
4.8/5  (34)
(34)
For the titration of 50.0 mL of 0.020 M aqueous salicylic acid with 0.020 M KOH(aq), calculate the pH after the addition of 55.0 mL of KOH(aq).For salycylic acid,PKa = 2.97.
(Multiple Choice)
4.9/5  (34)
(34)
Given: N2H5+(aq) N2(g).
How many electrons appear in the balanced half-reaction?
(Multiple Choice)
4.8/5  (42)
(42)
The following compounds are available as 0.10 M aqueous solutions. A) B) aniline C) D) E) methylamine F) G) H) I) triethylamine
Pick two solutions that could be used to prepare a buffer with a pH of ~ 10.8.More than one answer may be possible.
(Short Answer)
4.8/5  (37)
(37)
What is the proper cell diagram for the reaction
2AgCl(s)+ H2(g) 2Ag(s)+ 2H+(aq)+ 2Cl-(aq)
(Multiple Choice)
4.8/5  (39)
(39)
Calculate the [H+] in an aqueous solution that is 0.0755 M HF and 0.100 M NaF .The value of Ka for HF is 3.5 * 10-4.
(Multiple Choice)
4.9/5  (35)
(35)
Use the following diagram of a cell to answer questions 59-64: 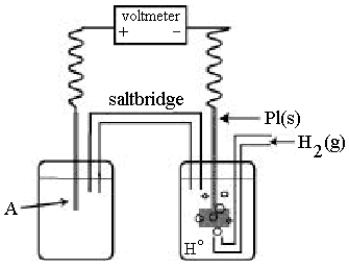 -In the cell shown above, A is a standard Zn2+/Zn electrode connected to a standard hydrogen electrode (SHE).If the voltmeter reading is -0.76 V,
Which electrode is negative?
-In the cell shown above, A is a standard Zn2+/Zn electrode connected to a standard hydrogen electrode (SHE).If the voltmeter reading is -0.76 V,
Which electrode is negative?
(Short Answer)
4.8/5  (44)
(44)
What is the pH at the half-stoichiometric point for the titration of 0.88 M HNO2(aq) with 0.10 M KOH(aq)? For HNO2,Ka = 4.3 * 10-4.
(Multiple Choice)
4.7/5  (42)
(42)
You have available the following reagents as 0.10 M aqueous solutions:NaOH,HCl,Phenol (pKa = 9.89),Aniline (pKb = 9.37),HNO2 (pKa = 3.37),CH3NH2 (pKb = 3.44).Which two reagents would you use to make a buffer with a pH of 10.5?
(Multiple Choice)
4.8/5  (38)
(38)
A certain weak acid has a Ka of 2.0 *10-5.What is the equilibrium constant for the reaction of this acid with a strong base?
(Short Answer)
4.8/5  (41)
(41)
A 0.0010 M solution of a weak acid, HA,With Ka = 2 * 10-10 produces [H3O+] < 10-6 M.Which of the following equations can be used to determine [H3O+]?
(Multiple Choice)
4.8/5  (34)
(34)
Calculate the equilibrium constant for the reaction that occurs when perchloric acid is added to the buffer B(aq)/BHCl(aq).The Kb of B is 3.4 * 10-5.
(Short Answer)
4.9/5  (36)
(36)
The solubility of all except which the following compounds increases as the pH of the solution decreases?
(Multiple Choice)
4.8/5  (44)
(44)
Consider the titration of 10.0 mL of 0.100 M (CH3)3N(aq)
with 0.100 M HClO4(aq).What is the formula of the main species in the solution after the addition of 10.0 mL of acid?
(Short Answer)
4.8/5  (40)
(40)
Showing 21 - 40 of 282
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)