Exam 6: Reactions
Exam 1: Atoms202 Questions
Exam 2: Molecules181 Questions
Exam 3: States of Matter287 Questions
Exam 4: Thermodynamics192 Questions
Exam 5: Equilibrium191 Questions
Exam 6: Reactions282 Questions
Exam 7: Kinetics87 Questions
Exam 8: The Main-Group Elements191 Questions
Exam 9: The D-Block Elements95 Questions
Exam 10: Nuclear Chemistry96 Questions
Exam 11: Organic Chemistry193 Questions
Select questions type
In a solution labeled "0.10 M HNO3," which of the following is correct?
(Multiple Choice)
4.7/5  (32)
(32)
For the cell diagram
Pt|H2(g)|H+(aq)mCo3+(aq),Co2+(aq)|Pt
write the reaction that occurs at the cathode.
(Short Answer)
4.9/5  (37)
(37)
The standard voltage of the cell
Pt|H2(g)|H+(aq)M Cl-(aq)|AgCl(s)|Ag(s)
Is +0.22 V at 25 C.Calculate the equilibrium constant for the reaction below.
2AgCl(s)+ H2(g) 2Ag(s)+ 2H+(aq)+ 2C-(aq)
(Multiple Choice)
4.8/5  (40)
(40)
Consider the following cell:
Zn(s)|Zn2+(aq,0.10 M)m Cu2+(aq,0.10 M)|Cu(s)
At equilibrium,what is the concentration of Cu2+(aq)?
(Short Answer)
4.7/5  (32)
(32)
In a working electrochemical cell (+ cell voltage),the cations in the salt bridge move toward the cathode.
(True/False)
4.7/5  (31)
(31)
Which of the following produces the strongest conjugate base?
(Multiple Choice)
4.9/5  (31)
(31)
If 50.0 mL of 0.22 M NaCl(aq)is mixed with 50.0 mL of 0.0050 M AgNO3(aq),will AgCl(s)precipitate? Ksp(AgCl)= 1.6 × 10-10.
(Essay)
4.7/5  (34)
(34)
The curve for the titration of 50.0 mL of 0.0200 M C6H5COOH(aq)
with 0.100 M NaOH(aq) is given below.What are the main species in the solution after 7.5 mL of base have been added? 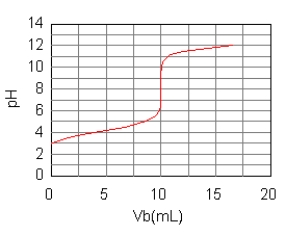
(Short Answer)
4.8/5  (36)
(36)
If the value of Kb for pyridine is 1.8 *10-9, calculate the equilibrium constant for
C5H5NH+(aq)+ H2O(l) C5H5N(aq)+ H3O+(aq)
(Multiple Choice)
4.9/5  (33)
(33)
Both H2O and OH-can act as a Brønsted acid and a Brønsted base but not as a Lewis acid.
(True/False)
4.8/5  (39)
(39)
The half-reaction that occurs at cathode when 1 M AgNO3(aq) is electrolyzed is __________________.
(Short Answer)
4.9/5  (36)
(36)
What is the pH of an aqueous solution that is 0.20 M HNO2 (Ka = 4.3 * 10-4) and 0.20 M NaNO2?
(Multiple Choice)
4.8/5  (43)
(43)
When KI(aq)is electrolyzed at a concentration of 1 M, the product at the anode is I2.
(True/False)
4.7/5  (32)
(32)
When a sample of an unknown metal is dropped into 1 M H+(aq) under standard conditions,bubbles are observed.The unknown metal could be silver.
(True/False)
4.8/5  (41)
(41)
The standard potential of the Cu2+/Cu electrode is +0.34 V and the standard potential of the cell
Ag(s)|AgCl(s)|Cl-(aq)M Cu2+(aq)|Cu(s)
Is +0.12 V.What is the standard potential of the AgCl/Ag,Cl- electrode?
(Multiple Choice)
4.9/5  (33)
(33)
Showing 101 - 120 of 282
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)