Exam 6: Reactions
Exam 1: Atoms202 Questions
Exam 2: Molecules181 Questions
Exam 3: States of Matter287 Questions
Exam 4: Thermodynamics192 Questions
Exam 5: Equilibrium191 Questions
Exam 6: Reactions282 Questions
Exam 7: Kinetics87 Questions
Exam 8: The Main-Group Elements191 Questions
Exam 9: The D-Block Elements95 Questions
Exam 10: Nuclear Chemistry96 Questions
Exam 11: Organic Chemistry193 Questions
Select questions type
The pH of 0.10 M solution and 0.35 M solution of NaHCO3(aq)must differ for each solution.
(True/False)
4.9/5  (37)
(37)
The amino acid alanine, HOOC-CH(CH3)NH3+,Has Ka1 = 4.5 *10-3 and Ka2 = 1.4 * 10-10.Calculate (HOOC-CH(CH3)NH3+)At pH 3.
(Multiple Choice)
4.8/5  (36)
(36)
The following 0.1 M aqueous solutions are arranged in order of increasing pH, with the highest pH on the far right.
Which one of the following 0.10 M aqueous solutions should be placed in the empty box?
(Multiple Choice)
4.8/5  (38)
(38)
The pH of 0.10 M pyridine(aq) is 9.13.What is the value of Kb for pyridine?
(Multiple Choice)
4.9/5  (31)
(31)
Which of the following occurs when HCl(aq), Cu(s),And Fe(s)Are mixed under standard conditions?
(Multiple Choice)
4.8/5  (36)
(36)
For a solution labeled "0.10 M H2SO3(aq)," pKa1 = 1.81 and pKa2 = 6.91, which of the following is true?
(Multiple Choice)
4.8/5  (39)
(39)
Consider the following cell:
Zn(s)|Zn2+(aq,0.200 M)M H+(aq,?)|H2(g,1.00 atm)|Pt
If E = +0.66 V and E 10 = +0.76 V at 25 C,
Calculate the concentration of H+ in the cathode cell compartment.
(Multiple Choice)
4.9/5  (42)
(42)
What is the molarity of OH- in a 0.0018 M calcium hydroxide solution?
(Multiple Choice)
4.8/5  (45)
(45)
Which of the following water-insoluble salts is more soluble in 1.0 M HClO4(aq)?
(Multiple Choice)
4.9/5  (38)
(38)
The titration curve for the titration of 0.100 M Na2CO3(aq) with 0.100 M HClO4(aq)
Is: 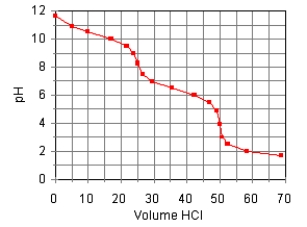 Estimate pKb1.
Estimate pKb1.
(Multiple Choice)
4.8/5  (39)
(39)
Which of the following occurs when HNO3(aq), Cu(s), And Pt(s)Are mixed under standard conditions?
(Multiple Choice)
4.8/5  (27)
(27)
How many moles of KOH(s)must be added to 1.00 L of 0.782 M HF(aq)to produce a buffer with a pH = 4.00?
(Short Answer)
4.9/5  (26)
(26)
The amino acid methionine, HOOC-CH(CH2CH2SCH3)NH3+,Has pKa1 = 2.2 and pKa2 = 9.1.If this amino acid is represented by H2L+,The major species at pH 6 is
(Multiple Choice)
4.9/5  (36)
(36)
If E for the following cell is 0.36 V at 25 C
Pb(s)|PbSO4(s)|SO42-(aq,0.60 M)M H+(aq,0.70 M)|H2(g,192.5 kPa)|Pt
How is the Nernst equation for the cell properly expressed at this temperature?
(Multiple Choice)
4.8/5  (30)
(30)
Calculate the ratio of the molarities of CO32- and HCO3-ions required to achieve buffering at pH = 9.0 .For H2CO3,PKa1 = 6.37,And pKa2 = 10.00.
(Multiple Choice)
4.9/5  (37)
(37)
Showing 81 - 100 of 282
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)