Exam 6: Reactions
Exam 1: Atoms202 Questions
Exam 2: Molecules181 Questions
Exam 3: States of Matter287 Questions
Exam 4: Thermodynamics192 Questions
Exam 5: Equilibrium191 Questions
Exam 6: Reactions282 Questions
Exam 7: Kinetics87 Questions
Exam 8: The Main-Group Elements191 Questions
Exam 9: The D-Block Elements95 Questions
Exam 10: Nuclear Chemistry96 Questions
Exam 11: Organic Chemistry193 Questions
Select questions type
Consider the following cell:
Ag(s)|Ag+(aq,0.100 M)mAg+(aq,0.100 M)|Ag(s)
What is the voltage of this cell?
Free
(Multiple Choice)
4.8/5  (41)
(41)
Correct Answer:
A
If (HSO3-) = 0.45 at pH 7.0,What are (H2SO3)And (SO32-)At this pH? For H2SO3,PKa1 and pKa2 are 1.81 and 6.91,Respectively.
Free
(Multiple Choice)
4.8/5  (33)
(33)
Correct Answer:
D
What is the pH of an aqueous solution that is 0.10 M HCOOH (Ka =1.8 * 10-4) and 0.10 M NaHCO2?
Free
(Multiple Choice)
4.9/5  (32)
(32)
Correct Answer:
B
The pH of 0.80 M benzenesulfonic acid is 0.51 .What is the percentage ionization of benzenesulfonic acid?
(Multiple Choice)
4.9/5  (44)
(44)
True or false: the pH of 0.10 M and 0.40 M NaHCO3(aq) solutions is 8.31 for both?
(True/False)
4.8/5  (44)
(44)
What is the pH at the half-stoichiometric point for the titration of 0.22 M HNO2(aq) with 0.10 M KOH(aq)? For HNO2,Ka = 4.3 * 10-4.
(Multiple Choice)
4.8/5  (42)
(42)
Use the following diagram of a cell to answer questions 59-64: 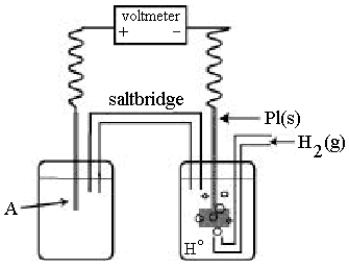 -Using the cell shown above, A is a standard Ag+/Ag electrode connected to a standard hydrogen electrode (SHE).When the voltmeter reading is -0.80 V,
Which half-reaction occurs in the left-hand cell compartment?
-Using the cell shown above, A is a standard Ag+/Ag electrode connected to a standard hydrogen electrode (SHE).When the voltmeter reading is -0.80 V,
Which half-reaction occurs in the left-hand cell compartment?
(Multiple Choice)
4.9/5  (46)
(46)
A buffer contains equal concentrations of NH3(aq) and NH4Cl(aq).What is the pH of the buffer? (Kb (NH3)= 1.8 * 10-5)
(Multiple Choice)
4.8/5  (29)
(29)
What is the value of E for the following reaction?
2H+(aq,1.00 M)+ 2e- H2(g,1.00 atm)
(Short Answer)
4.8/5  (44)
(44)
Which of the following 0.10 M aqueous solutions has the lowest pH?
(Multiple Choice)
4.8/5  (42)
(42)
The titration curve for the titration of 0.100 M Na2CO3(aq) with 0.100 M HClO4(aq)
Is: 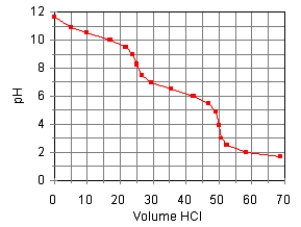 The main species in the solution after the addition of 35 mL of HClO4 are
The main species in the solution after the addition of 35 mL of HClO4 are
(Multiple Choice)
4.8/5  (30)
(30)
If (HSO3-) = 0.83 at pH 2.5,What are (H2SO3)And (SO32-)
At this pH? For H2SO3,PKa1 and pKa2 are 1.81 and 6.91,Respectively.
(Multiple Choice)
4.9/5  (33)
(33)
For the cell diagram
Cd(s)|CdSO4(aq)|Hg2SO4|Hg(l)
What reaction occurs at the cathode?
(Multiple Choice)
4.8/5  (33)
(33)
Showing 1 - 20 of 282
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)