Exam 6: Reactions
Exam 1: Atoms202 Questions
Exam 2: Molecules181 Questions
Exam 3: States of Matter287 Questions
Exam 4: Thermodynamics192 Questions
Exam 5: Equilibrium191 Questions
Exam 6: Reactions282 Questions
Exam 7: Kinetics87 Questions
Exam 8: The Main-Group Elements191 Questions
Exam 9: The D-Block Elements95 Questions
Exam 10: Nuclear Chemistry96 Questions
Exam 11: Organic Chemistry193 Questions
Select questions type
Calculate E for the following cell.
Zn(s)|Zn2+(aq)MCl-(aq)|AgCl(s)|Ag(s)
(Multiple Choice)
4.9/5  (40)
(40)
For the reduction of Cu2+ by Zn,
Go = -212 kJ/mol and Eo = +1.10 V.If the coefficients in the chemical equation for this reaction are multiplied by 2, Go = -424 kJ/mol.This means Eo = +2.20 V.
(True/False)
4.7/5  (31)
(31)
At the stoichiometric point in the titration of 0.260 M CH3NH2(aq) with 0.260 M HCl(aq),
(Multiple Choice)
4.7/5  (42)
(42)
The standard potential of the cell
Ag(s)|AgCl(s)MCl-(aq|Cu2+(aq)|Cu(s)
Is +0.12 V at 25 C.If the standard potential of the Cu2+/Cu couple is +0.34 V,
Calculate the standard potential of the AgCl/Ag,Cl-couple.
(Multiple Choice)
4.7/5  (38)
(38)
In a solution labeled "0.0018 M barium hydroxide" what is the molarity of OH-?
(Multiple Choice)
4.7/5  (28)
(28)
In the titration of 0.300 M CH3NH2(aq)with 0.300 M HCl(aq),what is the formula of the major species and what is its concentration at the equivalence point?
(Essay)
4.9/5  (39)
(39)
Write the charge balance equation for a dilute aqueous solution of KOH.
(Multiple Choice)
4.8/5  (37)
(37)
The Ksp for mercury(I) iodide is 1.2 *10-28.What is the solubility of mercury(I) Iodide?
(Multiple Choice)
4.9/5  (32)
(32)
Galvanized iron is protected from corrosion because zinc reduces any Fe2+ formed.
(True/False)
5.0/5  (43)
(43)
Calculate the hydroxide ion concentration for an aqueous solution that has a pH of 3.45.
(Multiple Choice)
4.8/5  (41)
(41)
Calculate the equilibrium constant for the reaction that occurs when sodium hydroxide is added to the buffer HA(aq)/NaA(aq).The Ka of HA is 4.1 * 10-5.
(Short Answer)
4.9/5  (43)
(43)
Bond polarity tends to dominate the trend of acid strengths for binary acids of elements of the same period.
(True/False)
4.9/5  (44)
(44)
How many moles of O2(g) are produced by electrolysis of Na2SO4(aq)
If 0.120 A is passed through the solution for 65.0 min?
(Multiple Choice)
5.0/5  (43)
(43)
What is the pH at the stoichiometric point for the titration of 0.100 M CH3COOH(aq) with 0.100 M KOH(aq)? The value of Ka for acetic acid is 1.8 * 10-5.
(Multiple Choice)
4.7/5  (33)
(33)
Use the following diagram of a cell to answer questions 59-64: 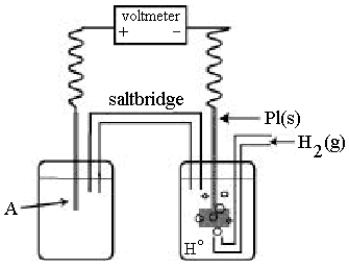 -In the cell shown above,A is a standard Ag+/Ag electrode connected to a standard hydrogen electrode (SHE).If the voltmeter reading is +0.80 V,which electrode is negative?
-In the cell shown above,A is a standard Ag+/Ag electrode connected to a standard hydrogen electrode (SHE).If the voltmeter reading is +0.80 V,which electrode is negative?
(Short Answer)
4.8/5  (37)
(37)
The amino acid alanine,HOOC-CH(CH3)NH3+,Has Ka1 = 4.5 * 10-3 and Ka2 = 1.4 * 10-10.Calculate (-OOC-CH(CH3)NH3+)At pH 10.
(Multiple Choice)
5.0/5  (40)
(40)
At the stoichiometric point in the titration of 0.130 M HCOOH(aq) with 0.130 M KOH(aq),
(Multiple Choice)
4.9/5  (36)
(36)
The following 0.1 M aqueous solutions are arranged in order of increasing pH, with the highest pH on the far right.
Which one of the following 0.10 M aqueous solutions should be placed in the empty box?
(Multiple Choice)
4.8/5  (45)
(45)
If the standard potential for Ti3+(aq)/Ti2+(aq) is -0.37 V and the standard potential for Ti2+(aq)/Ti(s)Is -1.63 V,Calculate the standard potential for
Ti3+(aq)+ 3e- Ti(s).
(Multiple Choice)
4.8/5  (41)
(41)
Showing 41 - 60 of 282
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)