Exam 6: Reactions
Exam 1: Atoms202 Questions
Exam 2: Molecules181 Questions
Exam 3: States of Matter287 Questions
Exam 4: Thermodynamics192 Questions
Exam 5: Equilibrium191 Questions
Exam 6: Reactions282 Questions
Exam 7: Kinetics87 Questions
Exam 8: The Main-Group Elements191 Questions
Exam 9: The D-Block Elements95 Questions
Exam 10: Nuclear Chemistry96 Questions
Exam 11: Organic Chemistry193 Questions
Select questions type
Which of the following mixtures gives a buffer with a pH less than 7.0? For acetic acid, Ka = 1.8 * 10-5 and for NH3,Kb = 1.8 *10 -5.
(Multiple Choice)
4.9/5  (32)
(32)
The following 0.1 M aqueous solutions are arranged in order of increasing pH, with the highest pH on the far right.
Which one of the following 0.10 M aqueous solutions should be placed in the empty box?
(Multiple Choice)
4.7/5  (40)
(40)
The standard potential of the Cu2+/Cu electrode is +0.34 V and the standard potential of the cell
Pb(s)|Pb2+(aq)M Cu2+(aq)|Cu(s)
Is +0.47 V.What is the standard potential of the Pb2+/Pb electrode?
(Multiple Choice)
4.9/5  (29)
(29)
The pH of a 0.0050 M aqueous solution of calcium hydroxide is
(Multiple Choice)
4.7/5  (34)
(34)
Calculate the equilibrium constant for the reaction that occurs when nitric acid is added to the buffer HA(aq)/NaA(aq).The Ka of HA is 1.2 * 10 -5.
(Short Answer)
4.9/5  (37)
(37)
When CaO(s) is dissolved in water,Which of the following is true?
(Multiple Choice)
4.8/5  (42)
(42)
The fractional composition diagram for the amino acid alanine is given below. 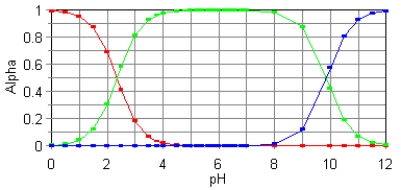 Write the structure of the dominant species at pH 1,6,and 12,respectively.
Write the structure of the dominant species at pH 1,6,and 12,respectively.
(Essay)
4.9/5  (32)
(32)
The titration curve for the titration of 0.100 M Na2CO3(aq) with 0.100 M HClO4(aq)
Is: 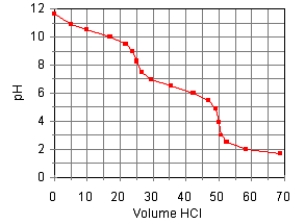 Estimate pKb2.
Estimate pKb2.
(Multiple Choice)
4.8/5  (43)
(43)
What is the pH at the stoichiometric point for the titration of 0.26 M CH3NH2(aq) with 0.26 M HClO4(aq)? For CH3NH2,Kb = 3.6 * 10-4.
(Multiple Choice)
4.9/5  (32)
(32)
The following compounds are available as 0.10 M aqueous solutions. A) B) aniline C) D) E) methylamine F) G) H) I) triethylamine
Pick two solutions that could be used to prepare a buffer with a pH of ~ 4.
(Short Answer)
4.8/5  (37)
(37)
Given: S2O42-(aq) SO32-(aq),Basic solution. How many electrons appear in the balanced half-reaction?
(Multiple Choice)
4.9/5  (40)
(40)
Calculate the value of the equilibrium constant for the reaction.
AgCl(s)+ 2NH3(aq)
Ag(NH3)2+(aq)+ Cl-(aq)
Given Ksp = 1.6 * 10-10 for silver chloride and Kf = 1.6 * 107 for the ammonia complex of Ag+ ions,
Ag(NH3)2+.
(Multiple Choice)
4.8/5  (42)
(42)
If 10.0 mmol of sodium hydroxide is added to 1.00 L of a buffer that is 0.200 M HClO(aq) and 0.155 M NaHClO(aq),The final concentrations of HClO(aq)
And ClO-(aq),Respectively,Are
(Multiple Choice)
4.8/5  (37)
(37)
What is the pKa of the conjugate acid of hydrazine,given that the pKb of hydrazine is 5.77? Write the formula of the conjugate acid of hydrazine.
(Short Answer)
4.9/5  (32)
(32)
Write the proper cell diagram for the following reaction:
2AuCl(s)+ H2(g) 2Au(s)+ 2H+(aq)+ 2Cl-(aq)
(Multiple Choice)
4.9/5  (35)
(35)
A buffer contains equal concentrations of a weak acid, HA,And its conjugate base,A-.If the value of Ka for HA is 1.0 * 10-9,What is the pH of the buffer?
(Multiple Choice)
4.8/5  (43)
(43)
Which of the following 0.10 M aqueous solutions gives the lowest pH?
(Multiple Choice)
4.9/5  (46)
(46)
Calculate the equilibrium concentration of sulfurous acid in a solution labeled "0.100 M H2SO3(aq)" if pKa1 = 1.81, and pKa2 = 6.91.
(Multiple Choice)
4.8/5  (43)
(43)
Sodium is produced by electrolysis of molten sodium chloride .What are the products at the anode and cathode,Respectively?
(Multiple Choice)
4.8/5  (36)
(36)
Showing 61 - 80 of 282
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)