Exam 6: Reactions
Exam 1: Atoms202 Questions
Exam 2: Molecules181 Questions
Exam 3: States of Matter287 Questions
Exam 4: Thermodynamics192 Questions
Exam 5: Equilibrium191 Questions
Exam 6: Reactions282 Questions
Exam 7: Kinetics87 Questions
Exam 8: The Main-Group Elements191 Questions
Exam 9: The D-Block Elements95 Questions
Exam 10: Nuclear Chemistry96 Questions
Exam 11: Organic Chemistry193 Questions
Select questions type
What is the main factor that directly determines the pH of any buffer?
(Essay)
4.7/5  (41)
(41)
Use the following to answer questions 55-58: 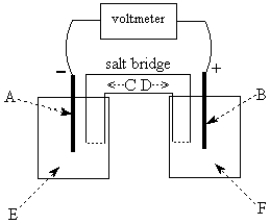 -The galvanic cell shown above uses the half-cells Pb2+/Pb and Zn2+/Zn,and a salt bridge containing KCl(aq).The voltmeter gives a positive voltage reading.The electrode B could be inert platinum metal or zinc.
-The galvanic cell shown above uses the half-cells Pb2+/Pb and Zn2+/Zn,and a salt bridge containing KCl(aq).The voltmeter gives a positive voltage reading.The electrode B could be inert platinum metal or zinc.
(True/False)
4.8/5  (40)
(40)
Choose the effective pH range of an aniline/anilinium chloride buffer .
The value of the Kb for aniline is 4.3 * 10-10.
(Multiple Choice)
4.8/5  (40)
(40)
If pKa1 and pKa2 for H2CO3 are 6.37 and 10.25, respectively,Calculate the equilibrium constant for the reaction below:
H2CO3(aq)+ 2H2O(l) 2H3O+(aq)+ CO32-(aq)
(Multiple Choice)
4.8/5  (40)
(40)
If a small amount of a strong base is added to buffer made up of a weak acid, HA,And the sodium salt of its conjugate base,NaA,The pH of the buffer solution does not change appreciably because
(Multiple Choice)
4.9/5  (39)
(39)
What is the pH of an aqueous solution that is 0.60 (CH3)3N (Kb = 6.5 * 10-5) and 0.95 M (CH3)3NHCl?
(Multiple Choice)
4.8/5  (32)
(32)
Use the following diagram of a cell to answer questions 59-64: 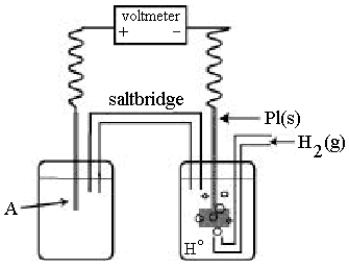 -In the cell shown above, A is a standard Zn2+/Zn electrode connected to a standard hydrogen electrode (SHE).If the voltmeter reading is -0.76 V,
Which half-reaction occurs in the left-hand cell compartment?
-In the cell shown above, A is a standard Zn2+/Zn electrode connected to a standard hydrogen electrode (SHE).If the voltmeter reading is -0.76 V,
Which half-reaction occurs in the left-hand cell compartment?
(Multiple Choice)
4.9/5  (39)
(39)
In the Daniell cell,the anode is zinc metal and the cathode is copper metal.When the cell operates,the anode gets smaller and the cathode gets larger.
(True/False)
4.8/5  (42)
(42)
Consider the following cell at standard conditions:
Zn(s)|Zn2+(aq)M Fe2+(aq)|Fe(s)
Calculate the value of Gr for the reaction that occurs when current is drawn from this cell.
(Multiple Choice)
4.8/5  (35)
(35)
If the standard potential for Cu2+(aq)/Cu+(aq) is 0.15 V and the standard potential for Cu2+(aq)/Cu(s)Is 0.34 V,Calculate the standard potential for
Cu+(aq)+ e- Cu(s).
(Multiple Choice)
4.7/5  (33)
(33)
A buffer solution contains 0.0200 M acetic acid and 0.0200 M sodium acetate.What is the pH after 2.0 mmol of NaOH are added to 1.00 L of this buffer? pKa = 4.75 for acetic acid.
(Multiple Choice)
4.7/5  (38)
(38)
If the molar solubility of the compound M2A3 is 7.0 * 10-6 M, what is the Ksp for this compound?
(Multiple Choice)
4.8/5  (42)
(42)
Which of the following aqueous solutions gives a pH greater than 7?
(Multiple Choice)
4.9/5  (34)
(34)
The pH of 0.010 M aniline(aq) is 8.32.What is the percentage aniline protonated?
(Multiple Choice)
4.9/5  (36)
(36)
How many seconds are required to produce 4.99 mg of chromium metal from an acidic solution of potassium dichromate,using a current of 0.234 A?
(Short Answer)
4.8/5  (38)
(38)
Use the following diagram of a cell to answer questions 59-64:  -In the cell shown above, A is a standard Ag+/Ag electrode connected to a standard hydrogen electrode (SHE).If the voltmeter reading is +0.80 V,
What is the equation for the cell reaction?
-In the cell shown above, A is a standard Ag+/Ag electrode connected to a standard hydrogen electrode (SHE).If the voltmeter reading is +0.80 V,
What is the equation for the cell reaction?
(Multiple Choice)
4.9/5  (42)
(42)
Consider the titration of 50.0 mL of 0.0200 M HClO(aq)with 0.100 M NaOH(aq).What is the formula of the main species in the solution after the addition of 10.0 mL of base?
(Short Answer)
4.8/5  (36)
(36)
Use the following diagram of a cell to answer questions 59-64:  -In the cell shown above, A is a standard Zn2+/Zn electrode connected to a standard hydrogen electrode (SHE).If the voltmeter reading is-0.76 V,
What is the equation for the cell reaction?
-In the cell shown above, A is a standard Zn2+/Zn electrode connected to a standard hydrogen electrode (SHE).If the voltmeter reading is-0.76 V,
What is the equation for the cell reaction?
(Multiple Choice)
4.9/5  (30)
(30)
Showing 161 - 180 of 282
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)