Exam 19: Chemical Thermodynamics
Exam 1: Introduction: Matter, energy, and Measurement163 Questions
Exam 2: Atoms, molecules, and Ions249 Questions
Exam 3: Chemical Reactions and Reaction Stoichiometry178 Questions
Exam 4: Reactions in Aqueous Solution178 Questions
Exam 5: Thermochemistry154 Questions
Exam 6: Electronic Structure of Atoms186 Questions
Exam 7: Periodic Properties of the Elements176 Questions
Exam 8: Basic Concepts of Chemical Bonding146 Questions
Exam 9: Molecular Geometry and Bonding Theories183 Questions
Exam 10: Gases175 Questions
Exam 11: Liquids and Intermolecular Forces124 Questions
Exam 12: Solids and Modern Materials84 Questions
Exam 13: Properties of Solutions160 Questions
Exam 14: Chemical Kinetics134 Questions
Exam 15: Chemical Equilibrium97 Questions
Exam 16: Acid-Base Equilibria139 Questions
Exam 17: Additional Aspects of Aqueous Equilibria116 Questions
Exam 18: Chemistry of the Environment126 Questions
Exam 19: Chemical Thermodynamics125 Questions
Exam 20: Electrochemistry113 Questions
Exam 21: Nuclear Chemistry178 Questions
Exam 22: Chemistry of the Nonmetals194 Questions
Exam 23: Transition Metals and Coordination Chemistry165 Questions
Exam 24: The Chemistry of Life: Organic and Biological Chemistry131 Questions
Select questions type
The quantity of energy gained by a system equals the quantity of energy gained by its surroundings.
Free
(True/False)
4.9/5  (37)
(37)
Correct Answer:
False
When a system is at equilibrium,________.
Free
(Multiple Choice)
4.8/5  (38)
(38)
Correct Answer:
D
For an isothermal process,ΔS = ________.
Free
(Multiple Choice)
4.8/5  (41)
(41)
Correct Answer:
B
The standard Gibbs free energy of formation of ________ is zero.
(a) H2O (l)
(b)O(g)
(c) CL2(g)
(Multiple Choice)
4.9/5  (31)
(31)
In the Haber process,ammonia gas is synthesized from nitrogen gas and hydrogen gas.ΔG° at 298 K for this reaction is -33.3 kJ/mol.The value of ΔG at 298 K for a reaction mixture that consists of 1.9 atm nitrogen gas,2.3 atm hydrogen gas,and 0.85 atm ammonia gas is ________.
(Multiple Choice)
4.8/5  (33)
(33)
For an isothermal process,the entropy change of the surroundings is given by the equation:
(Multiple Choice)
4.9/5  (40)
(40)
The value of ΔG° at 25 °C for the formation of POCl3 from its constituent elements, P2 (g)+ O2 (g)+ 3Cl2 (g)→ 2POCl3 (g)
Is ________ kJ/mol.
(Multiple Choice)
4.8/5  (40)
(40)
Calculate ΔG∘ (in kJ/mol)for the following reaction at 1 atm and 25 °C:
C2H6 (g)+ O2 (g)→ CO2 (g)+ H2O (l)(unbalanced)
ΔHf∘ C2H6 (g)= -84.7 kJ/mol; S∘ C2H6 (g)= 229.5 J/K ∙ mol;
ΔHf∘ CO2 (g)= -393.5 kJ/mol; S∘ CO2 (g)= 213.6 J/K ∙ mol;
ΔHf∘ H2O (l)= -285.8 kJ/mol; S∘H2O (l)= 69.9 J/K ∙ mol;
S∘O2 (g)= 205.0 J/K ∙ mol
(Short Answer)
4.8/5  (41)
(41)
The value of ΔH° for the formation of calcium chloride from its constituent elements, Ca (s)+ Cl2 (g)→ CaCl2 (s)
Is ________ kJ/mol.
(Multiple Choice)
4.7/5  (41)
(41)
The normal boiling point of methanol is 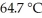 and the molar enthalpy of vaporization if 71.8 kJ/mol.The value of ΔS when 1.75 mol of
and the molar enthalpy of vaporization if 71.8 kJ/mol.The value of ΔS when 1.75 mol of 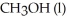 vaporizes at 64.7 °C is
vaporizes at 64.7 °C is 
(Multiple Choice)
4.7/5  (30)
(30)
The value of ΔG° at 181.0 °C for the formation of calcium chloride from calcium metal and chlorine gas is ________ kJ/mol.At 25.0 °C for this reaction,ΔH° is -795.8 kJ/mol,ΔG° is -748.1 kJ/mol,and 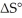 is
is 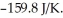
(Multiple Choice)
4.7/5  (40)
(40)
The value of ΔS° for the decomposition of phosphorous trichloride into its constituent elements, 2PCl3 (g)→ P2 (g)+ 3Cl2( g)
Is ________ J/K ∙ mol.
(Multiple Choice)
4.8/5  (28)
(28)
The value of ΔH° for the decomposition of gaseous sulfur trioxide to its component elements,
2SO3 (g)→ 2S (s,rhombic)+ 3O2 (g)
Is ________ kJ/mol.
(Multiple Choice)
4.8/5  (29)
(29)
The value of ΔH° for the oxidation of solid elemental sulfur to gaseous sulfur dioxide,
S (s,rhombic)+ O2 (g)→ SO2 (g)
Is ________ kJ/mol.
(Multiple Choice)
4.8/5  (30)
(30)
For the reaction C(s)+ 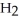 O(g)→ CO(g)+
O(g)→ CO(g)+ 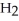 (g)
ΔH° = 133.3 kJ/mol and ΔS° = 121.6 J/K ∙ mol at 298 K.At temperatures greater than ________ °C this reaction is spontaneous under standard conditions.
(g)
ΔH° = 133.3 kJ/mol and ΔS° = 121.6 J/K ∙ mol at 298 K.At temperatures greater than ________ °C this reaction is spontaneous under standard conditions.
(Multiple Choice)
4.9/5  (37)
(37)
Phosphorous and chlorine gases combine to produce phosphorous trichloride.ΔG° at 298 K for this reaction is -642.9 kJ/mol.The value of ΔG at 298 K for a reaction mixture that consists of 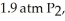
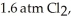 and
and 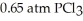 is ________.
is ________.
(Multiple Choice)
4.7/5  (32)
(32)
The value of ΔH° for the decomposition of phosphorous trichloride into its constituent elements, 2PCl3 (g)→ P2 (g)+ 3Cl2 (g)
Is ________ kJ/mol.
(Multiple Choice)
4.8/5  (33)
(33)
The value of ΔG° at 373 K for the oxidation of solid elemental sulfur to gaseous sulfur dioxide is ________ kJ/mol.At 298 K,ΔH° for this reaction is -269.9 kJ/mol,and ΔS° is +11.6 J/K.
(Multiple Choice)
4.7/5  (31)
(31)
Calculate ΔG° (in kJ/mol)for the following reaction at 1 atm and 25 °C:
C2H6 (g)+ O2 (g)→ CO2 (g)+ H2O (l)(unbalanced)
ΔGf° C2H6 (g)= -32.89 kJ/mol; ΔGf° CO2 (g)= -394.4 kJ/mol; ΔGf° H2O (l)= -237.13 kJ/mol
(Short Answer)
4.8/5  (33)
(33)
Showing 1 - 20 of 125
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)