Exam 14: Chemical Kinetics
Exam 1: Introduction: Matter, energy, and Measurement163 Questions
Exam 2: Atoms, molecules, and Ions249 Questions
Exam 3: Chemical Reactions and Reaction Stoichiometry178 Questions
Exam 4: Reactions in Aqueous Solution178 Questions
Exam 5: Thermochemistry154 Questions
Exam 6: Electronic Structure of Atoms186 Questions
Exam 7: Periodic Properties of the Elements176 Questions
Exam 8: Basic Concepts of Chemical Bonding146 Questions
Exam 9: Molecular Geometry and Bonding Theories183 Questions
Exam 10: Gases175 Questions
Exam 11: Liquids and Intermolecular Forces124 Questions
Exam 12: Solids and Modern Materials84 Questions
Exam 13: Properties of Solutions160 Questions
Exam 14: Chemical Kinetics134 Questions
Exam 15: Chemical Equilibrium97 Questions
Exam 16: Acid-Base Equilibria139 Questions
Exam 17: Additional Aspects of Aqueous Equilibria116 Questions
Exam 18: Chemistry of the Environment126 Questions
Exam 19: Chemical Thermodynamics125 Questions
Exam 20: Electrochemistry113 Questions
Exam 21: Nuclear Chemistry178 Questions
Exam 22: Chemistry of the Nonmetals194 Questions
Exam 23: Transition Metals and Coordination Chemistry165 Questions
Exam 24: The Chemistry of Life: Organic and Biological Chemistry131 Questions
Select questions type
The half-life of a first-order reaction is 13 min.If the initial concentration of reactant is 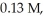 it takes
it takes  for it to decrease to 0.085 M.
for it to decrease to 0.085 M.
Free
(Multiple Choice)
4.9/5  (28)
(28)
Correct Answer:
C
The elementary reaction 2NO2 (g)→ 2NO (g)+ O2 (g)
Is second order in 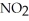 and the rate constant at 660 K is 5.23
and the rate constant at 660 K is 5.23 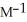
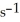 .The reaction half-life at this temperature when
.The reaction half-life at this temperature when 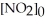 = 0.45 M is ________ s.
= 0.45 M is ________ s.
Free
(Multiple Choice)
4.8/5  (33)
(33)
Correct Answer:
E
Rates of reaction can be positive or negative.
Free
(True/False)
4.9/5  (35)
(35)
Correct Answer:
False
A second-order reaction has a half-life of 12 s when the initial concentration of reactant is 0.98 M.The rate constant for this reaction is ________ M-1s-1.
(Multiple Choice)
4.8/5  (38)
(38)
If a rate law is first order (reactant),doubling the reactant ________ the reaction rate.
(Short Answer)
4.9/5  (42)
(42)
The rate constant of a first-order process that has a half-life of 3.50 min is ________ s-1.
(Multiple Choice)
4.8/5  (30)
(30)
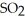
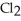 decomposes in the gas phase by the reaction SO2Cl2 (g)→ SO2 (g)+ Cl2 (g)
The reaction is first order in SO2Cl2 and the rate constant is 3.0 ×
decomposes in the gas phase by the reaction SO2Cl2 (g)→ SO2 (g)+ Cl2 (g)
The reaction is first order in SO2Cl2 and the rate constant is 3.0 × 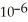
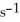 at 600 K.A vessel is charged with 3.6 atm of SO2Cl2 at 600 K.The partial pressure of SO2Cl2 at
at 600 K.A vessel is charged with 3.6 atm of SO2Cl2 at 600 K.The partial pressure of SO2Cl2 at 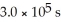 is ________ atm.
is ________ atm.
(Multiple Choice)
4.7/5  (36)
(36)
Of the following,________ will lower the activation energy for a reaction.
(Multiple Choice)
4.8/5  (39)
(39)
Fe and Mo are transition metals found in the cofactor active site of ________.
(Multiple Choice)
4.8/5  (39)
(39)
The reaction CH3-N≡C → CH3-C≡N
Is a first-order reaction.At 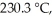
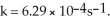 If
If 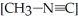 is 1.00 × 10-3 initially,
is 1.00 × 10-3 initially, 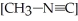 is ________ M after
is ________ M after 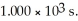
(Multiple Choice)
4.9/5  (33)
(33)
A compound decomposes by a first-order process.If 13% of the compound decomposes in 60 minutes,the half-life of the compound is ________ min.
(Multiple Choice)
4.7/5  (34)
(34)
At elevated temperatures,dinitrogen pentoxide decomposes to nitrogen dioxide and oxygen: 2N2O5(g)→ 4NO2 (g)+ O2 (g)
When the rate of formation of NO2 is 5.5 × 10-4 M/s,the rate of decomposition of N2O5 is ________ M/s.
(Multiple Choice)
4.9/5  (33)
(33)
What is the magnitude of k for a first-order reaction at 75.0 °C if 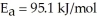 and rate constant is 1.35 ×
and rate constant is 1.35 × 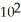
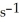 at 25.0 °C?
at 25.0 °C?
(Multiple Choice)
4.8/5  (36)
(36)
For the elementary reaction NO3 + CO → NO2 + CO2
The molecularity of the reaction is ________,and the rate law is 
(Multiple Choice)
4.7/5  (36)
(36)
Which of the following is correct regarding the overall order of the reaction?
(Multiple Choice)
4.8/5  (32)
(32)
The reaction 2NO2 → 2NO + O2
Follows second-order kinetics.At 300 °C,[NO2] drops from 0.0100 M to 0.00650 M in 100.0 s.The rate constant for the reaction is ________ M-1s-1.
(Multiple Choice)
4.7/5  (38)
(38)
The average rate of disappearance of A between 10 s and 20 s is ________ mol/s.
(Multiple Choice)
4.9/5  (33)
(33)
Showing 1 - 20 of 134
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)