Exam 15: Chemical Equilibrium
Exam 1: Introduction: Matter, energy, and Measurement163 Questions
Exam 2: Atoms, molecules, and Ions249 Questions
Exam 3: Chemical Reactions and Reaction Stoichiometry178 Questions
Exam 4: Reactions in Aqueous Solution178 Questions
Exam 5: Thermochemistry154 Questions
Exam 6: Electronic Structure of Atoms186 Questions
Exam 7: Periodic Properties of the Elements176 Questions
Exam 8: Basic Concepts of Chemical Bonding146 Questions
Exam 9: Molecular Geometry and Bonding Theories183 Questions
Exam 10: Gases175 Questions
Exam 11: Liquids and Intermolecular Forces124 Questions
Exam 12: Solids and Modern Materials84 Questions
Exam 13: Properties of Solutions160 Questions
Exam 14: Chemical Kinetics134 Questions
Exam 15: Chemical Equilibrium97 Questions
Exam 16: Acid-Base Equilibria139 Questions
Exam 17: Additional Aspects of Aqueous Equilibria116 Questions
Exam 18: Chemistry of the Environment126 Questions
Exam 19: Chemical Thermodynamics125 Questions
Exam 20: Electrochemistry113 Questions
Exam 21: Nuclear Chemistry178 Questions
Exam 22: Chemistry of the Nonmetals194 Questions
Exam 23: Transition Metals and Coordination Chemistry165 Questions
Exam 24: The Chemistry of Life: Organic and Biological Chemistry131 Questions
Select questions type
Which of the following expressions is the correct equilibrium-constant expression for the equilibrium between dinitrogen tetroxide and nitrogen dioxide?
N2O4 (g)  2NO2 (g)
2NO2 (g)
Free
(Multiple Choice)
4.9/5  (28)
(28)
Correct Answer:
B
Which of the following expressions is the correct equilibrium-constant expression for the reaction below?
HF (aq)+ H2O (l)  H3O+ (aq)+ F- (aq)
H3O+ (aq)+ F- (aq)
Free
(Multiple Choice)
4.9/5  (36)
(36)
Correct Answer:
D
The Keq for the equilibrium below is 50. H2 (g)+ I2 (g)  2HI (g)
What is the value of Keq for the following reaction?
2HI (g)
2HI (g)
What is the value of Keq for the following reaction?
2HI (g)  H2 (g)+ I2 (g)
H2 (g)+ I2 (g)
Free
(Multiple Choice)
4.7/5  (33)
(33)
Correct Answer:
C
The value of Keq for the equilibrium CO2 (g)+ 2H2 (g)  CH3OH (g)
Is 14.5 at 483 °C.What is the value of Keq for the equilibrium below?
1/2 CO2 + H2 (g)
CH3OH (g)
Is 14.5 at 483 °C.What is the value of Keq for the equilibrium below?
1/2 CO2 + H2 (g)  1/2 CH3OH (g)
1/2 CH3OH (g)
(Multiple Choice)
4.9/5  (30)
(30)
Consider the following reaction at equilibrium: 2CO2 (g)  2CO (g)+ O2 (g)ΔH° = -514 kJ
Le Châtelier's principle predicts that a decrease in temperature will ________.
2CO (g)+ O2 (g)ΔH° = -514 kJ
Le Châtelier's principle predicts that a decrease in temperature will ________.
(Multiple Choice)
4.7/5  (29)
(29)
The Keq for the equilibrium below is 50. H2 (g)+ I2 (g)  2HI (g)
What is the value of Keq for the following reaction?
2HI (g)
What is the value of Keq for the following reaction?  H2 (g)+
H2 (g)+  I2 (g)
I2 (g)  HI (g)
HI (g)
(Multiple Choice)
4.8/5  (32)
(32)
The expression of Keq for the following reaction will not include ________. A(g)+ B(g)  C(l)+ D(g)
C(l)+ D(g)
(Multiple Choice)
4.7/5  (34)
(34)
At elevated temperatures,molecular hydrogen and molecular bromine react to partially form hydrogen bromide: H2 (g)+ Br2 (g)  2HBr (g)
A mixture of 0.682 mol of H2 and 0.440 mol of Br2 is combined in a reaction vessel with a volume of
2HBr (g)
A mixture of 0.682 mol of H2 and 0.440 mol of Br2 is combined in a reaction vessel with a volume of 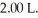 At equilibrium at 700 K,there are 0.546 mol of H2 present.At equilibrium,there are ________ mol of Br2 present in the reaction vessel.
At equilibrium at 700 K,there are 0.546 mol of H2 present.At equilibrium,there are ________ mol of Br2 present in the reaction vessel.
(Multiple Choice)
4.9/5  (36)
(36)
If the reaction quotient Q for a reaction is equal to the value of the equilibrium constant K for that reaction at a given temperature,then the reaction is at ________.
(Short Answer)
4.8/5  (37)
(37)
Hydrogen and iodine gas react to produce hydrogen iodide gas.What is the value of 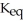 for this reaction if at equilibrium the concentrations of
for this reaction if at equilibrium the concentrations of 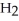 ,
, 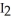 ,and HI were
,and HI were 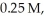
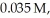 and
and 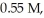 respectively?
respectively?
(Multiple Choice)
4.9/5  (34)
(34)
Carbon monoxide and chlorine gas react to produce COCl2 gas.The 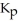 for the reaction is 1.49 × 108 at 100.0 °C: In an equilibrium mixture of the three gases,
for the reaction is 1.49 × 108 at 100.0 °C: In an equilibrium mixture of the three gases, 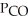 =
= 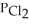 = 7.70 × 10-4 atm.The partial pressure of the product,phosgene (
= 7.70 × 10-4 atm.The partial pressure of the product,phosgene ( 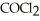 ),is ________ atm.
),is ________ atm.
(Multiple Choice)
4.8/5  (39)
(39)
At 200 °C,the equilibrium constant (Kp)for the conversion of NO to oxygen and nitrogen gas is 2.40 × 103.A closed vessel is charged with 36.1 atm of NO.At equilibrium,the partial pressure of O2 is ________ atm.
(Multiple Choice)
4.9/5  (34)
(34)
The Keq for the equilibrium below is 7.52 × 10-2 at 480.0 °C. 2Cl2 (g)+ 2H2O (g)  4HCl (g)+ O2 (g)
What is the value of Keq at this temperature for the following reaction?
8HCl (g)+ 2O2 (g)
4HCl (g)+ O2 (g)
What is the value of Keq at this temperature for the following reaction?
8HCl (g)+ 2O2 (g)  4Cl2 (g)+ 4H2O (g)
4Cl2 (g)+ 4H2O (g)
(Multiple Choice)
4.9/5  (40)
(40)
At 400 K,the equilibrium constant for the reaction Br2 (g)+ Cl2 (g)  2BrCl (g)
Is Kp = 7.0.A closed vessel at 400 K is charged with 1.00 atm of Br2 (g),1.00 atm of Cl2 (g),and 2.00 atm of BrCl (g).Use Q to determine which of the statements below is true.
2BrCl (g)
Is Kp = 7.0.A closed vessel at 400 K is charged with 1.00 atm of Br2 (g),1.00 atm of Cl2 (g),and 2.00 atm of BrCl (g).Use Q to determine which of the statements below is true.
(Multiple Choice)
4.7/5  (35)
(35)
In which of the following reactions would increasing pressure at constant temperature change the concentrations of reactants and products,based on Le Châteliers principle?
(Multiple Choice)
4.8/5  (23)
(23)
The equilibrium-constant expression depends on the ________ of the reaction.
(Multiple Choice)
4.8/5  (45)
(45)
Consider the following reaction at equilibrium: 2S
(g)+ C:\Users\User\Dropbox\Quizplus Parsing Documents\To Be Parsed\Not Complete Tables\Chemistry\TB1194,Chemistry The Central Science 14th Edition by Theodore E.Brown\TB1194,Chemistry The Central Science 14th Edition by Theodore E.Brown\Images\C10074 (15)_205.jpg
(g) C:\Users\User\Dropbox\Quizplus Parsing Documents\To Be Parsed\Not Complete Tables\Chemistry\TB1194,Chemistry The Central Science 14th Edition by Theodore E.Brown\TB1194,Chemistry The Central Science 14th Edition by Theodore E.Brown\Images\C10074 (15)_206.jpg
2S C:\Users\User\Dropbox\Quizplus Parsing Documents\To Be Parsed\Not Complete Tables\Chemistry\TB1194,Chemistry The Central Science 14th Edition by Theodore E.Brown\TB1194,Chemistry The Central Science 14th Edition by Theodore E.Brown\Images\C10074 (15)_207.jpg
(g)ΔH° = -99 kJ
Le Châtelier's principle predicts that a(n)increase in temperature will result in ________.
(Multiple Choice)
4.8/5  (27)
(27)
The equilibrium-constant expression for the reaction Ti (s)+ 2Cl2 (g)  TiCl4 (l)
Is given by
TiCl4 (l)
Is given by
(Multiple Choice)
4.7/5  (36)
(36)
If a reaction is endothermic,________ the reaction temperature results in an decrease in K.
(Short Answer)
4.8/5  (41)
(41)
If Reaction A + Reaction B = Reaction C,then Kc for Reaction C is ________.
(Essay)
4.8/5  (41)
(41)
Showing 1 - 20 of 97
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)