Exam 10: Gases
Exam 1: Introduction: Matter, energy, and Measurement163 Questions
Exam 2: Atoms, molecules, and Ions249 Questions
Exam 3: Chemical Reactions and Reaction Stoichiometry178 Questions
Exam 4: Reactions in Aqueous Solution178 Questions
Exam 5: Thermochemistry154 Questions
Exam 6: Electronic Structure of Atoms186 Questions
Exam 7: Periodic Properties of the Elements176 Questions
Exam 8: Basic Concepts of Chemical Bonding146 Questions
Exam 9: Molecular Geometry and Bonding Theories183 Questions
Exam 10: Gases175 Questions
Exam 11: Liquids and Intermolecular Forces124 Questions
Exam 12: Solids and Modern Materials84 Questions
Exam 13: Properties of Solutions160 Questions
Exam 14: Chemical Kinetics134 Questions
Exam 15: Chemical Equilibrium97 Questions
Exam 16: Acid-Base Equilibria139 Questions
Exam 17: Additional Aspects of Aqueous Equilibria116 Questions
Exam 18: Chemistry of the Environment126 Questions
Exam 19: Chemical Thermodynamics125 Questions
Exam 20: Electrochemistry113 Questions
Exam 21: Nuclear Chemistry178 Questions
Exam 22: Chemistry of the Nonmetals194 Questions
Exam 23: Transition Metals and Coordination Chemistry165 Questions
Exam 24: The Chemistry of Life: Organic and Biological Chemistry131 Questions
Select questions type
Two gases start to escape from a container and one of the gases effuses 1.25 times as fast as the other one.The two gases could have been ________.
Free
(Multiple Choice)
4.8/5  (37)
(37)
Correct Answer:
B
Of the following,________ is a valid statement of Charles' law.
Free
(Multiple Choice)
4.7/5  (32)
(32)
Correct Answer:
B
A closed-end manometer was attached to a vessel containing argon. The difference in the mercury levels in the two arms of the manometer was 9.60 cm.Atmospheric pressure was 783 mm Hg.The pressure of the argon in the container was ________ mm Hg.
(Multiple Choice)
4.7/5  (36)
(36)
At 500 °C,which of the following gases will have the greatest root-mean-square speed?
(Multiple Choice)
4.8/5  (39)
(39)
Molecular compounds of low molecular weight tend to be gases at room temperature.Which of the following is most likely not a gas at room temperature?
(Multiple Choice)
4.8/5  (32)
(32)
Which of the following gases would have a similar rate of effusion to CO at 350 K?
(Multiple Choice)
4.9/5  (27)
(27)
The thermal decomposition of potassium chlorate can be used to produce oxygen in the laboratory. 2KClO3 (s)→ 2KCl (s)+ 3O2 (g)
What volume (L)of O2 gas at 25 °C and 1.00 atm pressure is produced by the decomposition of 7.5 g of KClO3 (s)?
(Multiple Choice)
5.0/5  (38)
(38)
What volume (L)of HCl gas is required to react with an excess of sodium to generate 19.5 L of hydrogen gas at 1.31 atm and 51.0 °C?
(Short Answer)
4.8/5  (31)
(31)
Automobile air bags use the decomposition of sodium azide as their source of gas for rapid inflation: 2NaN3 (s)→ 2Na (s)+ 3N2 (g).
What mass (g)of NaN3 is required to provide 26.5 L of N2 at 22.0 °C and 1.10 atm?
(Multiple Choice)
4.7/5  (30)
(30)
The pressure exerted by 1.0 mol of gas in a 13 L flask at 22 °C is ________ kPa.
(Multiple Choice)
4.8/5  (34)
(34)
A 0.133 mol sample of gas in a 525 mL container has a pressure of 312 torr.The temperature of the gas is ________ °C.
(Multiple Choice)
4.9/5  (38)
(38)
A helium balloon is filled to a volume of 27.7 L at 300 K.What will the volume of the balloon become if the balloon is heated to raise the temperature to 392 K?
(Multiple Choice)
4.9/5  (44)
(44)
CO (5.00 g)and CO2 (5.00 g)were placed in a 750.0 mL container at 50.0 °C.The partial pressure of CO2 in the container was ________ atm.
(Multiple Choice)
4.9/5  (30)
(30)
Using the van der Waals equation,the pressure in a 22.4 L vessel containing 1.00 mol of neon gas at 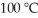 is ________ atm. (a = 0.211 L2-atm/mol2,b = 0.0171 L/mol)
is ________ atm. (a = 0.211 L2-atm/mol2,b = 0.0171 L/mol)
(Multiple Choice)
4.9/5  (34)
(34)
CO (5.00 g)and CO2 (5.00 g)were placed in a 750.0 mL container at 50.0 °C.The total pressure in the container was ________ atm.
(Multiple Choice)
4.8/5  (30)
(30)
A sample of gas (24.2 g)initially at 4.00 atm was compressed from 8.00 L to 2.00 L at constant temperature.After the compression,the gas pressure was ________ atm.
(Multiple Choice)
4.9/5  (40)
(40)
One significant difference between gases and liquids is that ________.
(Multiple Choice)
4.9/5  (49)
(49)
What is the volume (in m3)of a 0.25 mol of an unknown gas at a pressure of 545.3 mm Hg and 15 °C?
(Multiple Choice)
4.8/5  (29)
(29)
Arrange the following gases in order of increasing average molecular speed at 25 °C. Cl2,O2,F2,N2
(Multiple Choice)
4.8/5  (35)
(35)
Showing 1 - 20 of 175
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)